Aragen Oncology: Preclinical Testing Services
- Experienced and skilled team of research scientists to support preclinical research programs from study design through execution and data analysis
- Specialized equipment to conduct innovative research
- State-of-the art facilities in San Francisco Bay Area, CA, USA
- Each facility is AAALAC-accredited, OLAW-assured with DEA licenses
Expertise in in vivo as well as ex vivo analyses in murine translational oncology models to evaluate:
- Immune checkpoint inhibitors
- Bispecific antibodies
- Adoptive T cell transfer
- Chimeric antigen receptor (CAR) T-cell therapy
- Small molecule drugs
- Biologics
- Antibody treatment
Supporting Preclinical Oncology Testing Services
- Efficacy Assessment: IND-enabling
- Xenograft Tumor Models
- Orthotopic Tumor Models
- Immuno-Oncology Tumor Models
- Pharmacokinetics and Pharmacodynamics
- Immunological Assessment (FACS/IHC)
- Mechanism of Action (MOA) Assessment (Polymerase Chain Reaction (PCR), Western Blot (WB) etc
- End-to-End Histopathology, all major organs
- Non-GLP Clinical Safety Assessment
Furthermore, Aragen offers a broad range of preclinical oncology models and services, encompassing human cancer cell line xenograft (CDX), obtained by commercial vendors like Jackson Lab, immuno-oncology (IO). The IO efficacy testing service includes both innovative immune cell therapy and the assessment of proof of concept using syngeneic tumor models. We also provide a comprehensive range of additional services including clinically relevant biomarkers, pharmacokinetic-pharmacodynamic (PK/PD) analysis, histology analysis as well as genomic, proteomic biomarker analysis. The determination of Maximum Tolerated Dose (MTD) is available for both non-tumor and tumor-bearding mice can also be determined.
Human Cell Line Xenograft Models
Xenograft models are playing a crucial role in evaluating New Chemical Entities (NCEs) and New Biological Entities (NBEs) as promising anti-cancer agents. Rely on our experience in this field to support your research efforts and contribute to the progression of innovative treatments.
- Melanoma (A375)
- Ovarian cancer (SK-OV-3)
- Colon cancer (KM-12)
- Lung Cancer (A549)
- Breast Cancer (MDA-MB-231)
- Prostate Cancer (PC-3 & DU-145)
- Glioblastoma (U-87MG)
- Multiple Myeloma (MM.1S & RPMI 8226)
Tumor implantation sites
- Subcutaneous Model
- Disseminated Model
- Orthotopic Model
Note: For new models not previously established at Aragen, we recommend assessing the growth kinetics of in vivo models.
Syngeneic Tumor Models
Syngeneic models are routinely used in immune-oncology studies because they provide an in vivo system with normal immune function. Explore the advantages of utilizing our extensively characterized and checkpoint inhibitor-benchmarked syngeneic models to expedite the progress of your immuno-oncology drug discovery initiatives. Tumor models include, but are not limited to:
- Melanoma
- Breast Cancer
- Colon Cancer
- Brain Tumor
- Prostate Cancer
- Lung Cancer
- Pancreatic Cancer
Methodology
-
- Female Balb/C Mice were injected with RENCA cells (mouse melanoma cell line) in the flank region.
- Treatment with Cabozantinib was injected on Day (post implant).
Result
- A significant reduction in tumor volume and increased tumor growth inhibition was observed as compared with control grp.
Methodology
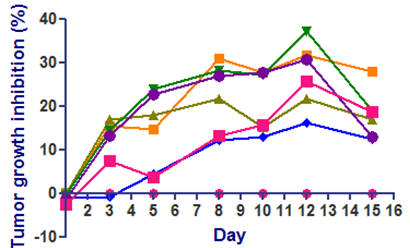
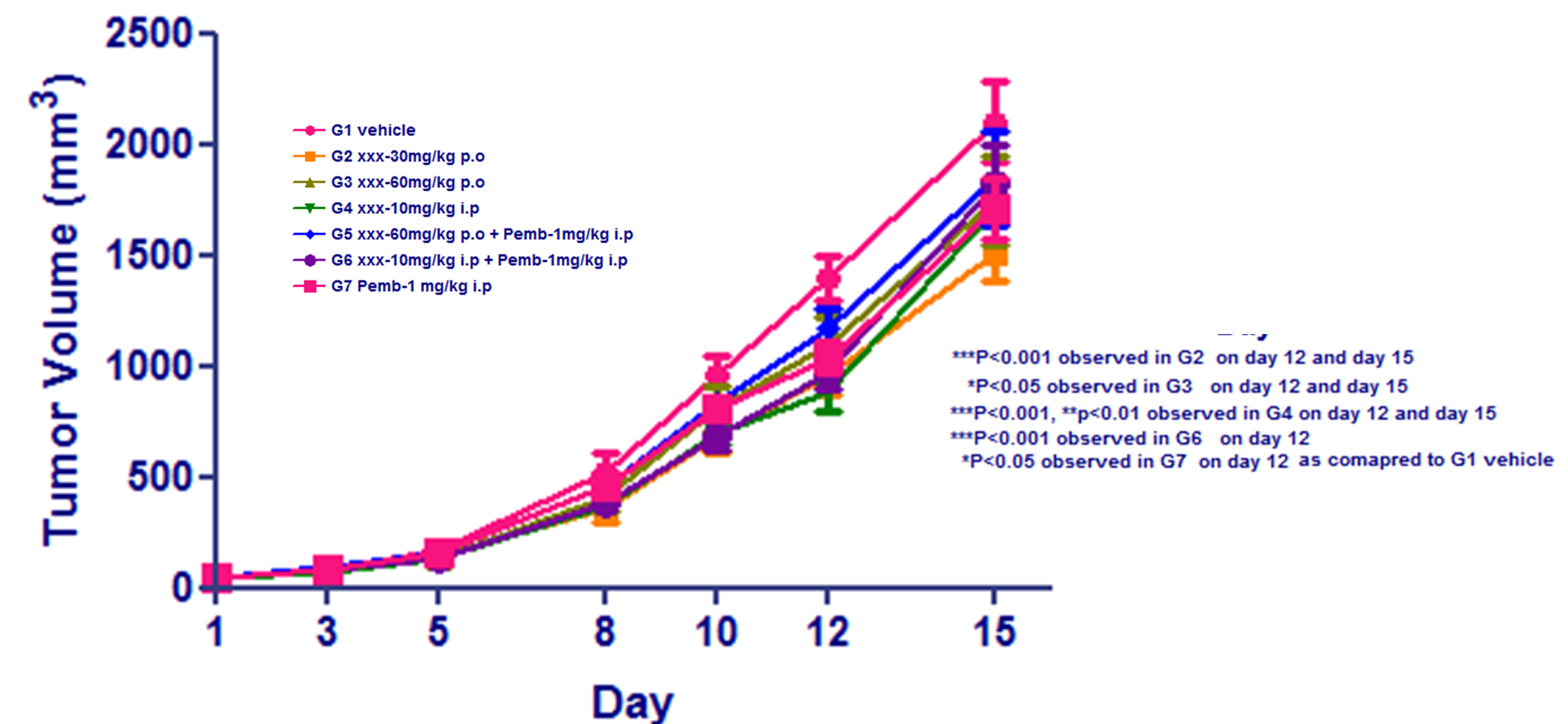
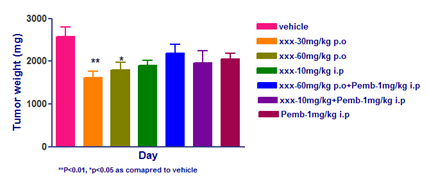
- Animals: Female C57BL/6 mice from Taconic (n=8 per group)
- Cell number: B16F10-mouse melanoma cell line; 1X106 cells in PBS was injected at flank region
- Reference compounds: Pembrolizumab- 1mg/kg; i.p; Q2D
Result
- XXX has moderately reduced the tumor volume compared to vehicle
- Pemrolozumab-alone or in combination with XXX has not reduced B16F10 tumor burden.
- The Pebrolozumab has reported elsewhere to have very minimal effect on B16F10 tumors
Humanized Immuno-Oncology Models
Aragen Bioscience is your dedicated partner in advancing your promising lead candidates and ensuring the success of your IND submission. Our specialized preclinical research services are designed to empower your research with precision and quality.
Syngeneic Mouse Models:
Syngeneic mouse models are your key to assessing the efficacy of immuno-oncology therapeutics that influence the immune system. With these models, we systematically evaluate crucial parameters and biomarkers, providing insights into therapeutic effectiveness, mechanism of action, and safety.
Human Immune System Models:
CAR-T cell therapy has been a momentous change, especially in the treatment of blood cancer. At Aragen bioscience, we offer highly customized preclinical evaluation of CAR-T therapy. Our experts meticulously oversee and prepare CAR-T cells, ensuring seamless adoptive transfer for efficacy assessment and non-GLP safety evaluation.
Bispecific Antibodies are a promising therapeutic approach, and we frequently assess their potential using severe immunodeficient models reconstituted with human PBMCs. We can also procure specialized HIS mice for evaluating other immunotherapeutics that utilize NK or Myeloid function.
Key Highlights
- Our team is comprised of experienced PhD scientists with diverse interdisciplinary backgrounds, along with histology experts.
- We provide a collaborative and knowledge-based advisory approach, guiding you from study design to execution in a timely manner.
- We understand your unique research requirements and assist in selecting the most suitable mouse models for your immunotherapy focus.
- Our study designs are guided by scientific principles, emphasizing the production of reproducible quality data.
- We leverage bioluminescent imaging (IVIS) to monitor tumor progression in live animals.
- Our Cell Biology Services includes a wide range of immune system characterizations, from immunophenotyping to cytokine analysis, ensuring comprehensive insights.
Experience the Convenience of Aragen:
Based in Morgan Hill, CA, we offer rapid domestic shipping for temperature-sensitive samples and therapeutics. We are excited to collaborate with you, contribute to your research endeavors, and pave the way for your success.
Your research deserves the excellence and precision that only Aragen bioscience can deliver.
Aragen offers innovative immunological research models such as Peripheral blood mononuclear cells (PBMC) Humanized mice models. These humanized mouse models have been engrafted with human cells to better reflect human immune responses.
Peripheral blood mononuclear cells (PBMC) Humanized mice models
(CAR) T-Cell Therapeutic Evaluations
The success of CART-cell therapy in hematologic malignancies renewed efforts to also use this technology against solid tumors. However, this strategy has had several challenges, including targeted delivery, penetration of therapeutics to tumor cells, immunogenicity, short- and long-term toxicity and safety, and efficacy of CART-cells in humans. To address these challenges, several animal models have been utilized to screen potential CART-cell therapeutics as part of the IND-enabling process. Aragen has extensive experience in CART evaluations of both liquid and solid tumors from intravenous adoptive transfer of CART cells through in vivo luminescent monitoring and ex vivo FACS analysis.
Human Peripheral Mononuclear Cells (PBMC) Model
Methodology
- Nude mice were implanted with 22rv.1 tumor cells.
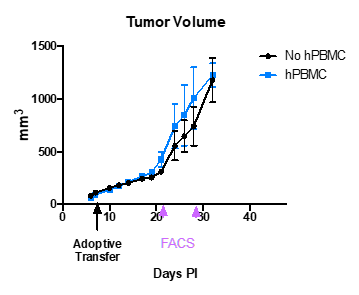
- On day 7 post tumor implantation (PI), when the average tumor was 100 mm^3, few animals were adoptively transferred with human PBMC.
- Tumor growth measured for 40 days PI.
- Ex vivo FACS analyses performed on tumors removed on Day 21 and Day 28
Results
- Human lymphocytes (CD3+ CD45+) were detected in blood sample of the mouse adoptively transferred with hPBMCs.
- CD3+ and CD45+ lymphocytes detected in blood of mice collected on both days 21 and 28.
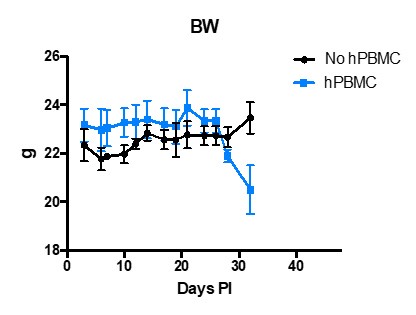
- Tumor growth (Tumor volume) and body weight (BW) with and without human PBMCs are shown in the following graphs.
- Mice that were adoptively transferred with hPBMCs developed GvHD around day 28 pi about 3 weeks after the adoptive transfer including substantial body weight loss.
Noninvasive In Vivo Imaging of Tumors
In vivo luminescence and fluorescence combined with X-rays imaging allows non-invasive monitoring of living mice longitudinally, offering real time insight into treatment efficacy, whole-body biodistribution, and target mechanisms. This poster demonstrates the development and characterization of several xenograft and syngeneic models for preclinical study applications at Aragen Bioscience.
Creation of Customized, Client Specific Study Designs in Numerous Mouse Tumor Models
- Luminescent Signals
-
- Syngeneic and xenograft cell lines
- Generated at Aragen
- Purchased/licensed by Aragen
- Client provided
- Commercially available
- Fluorescent reagents
-
- Commercially available targeted and activatable fluorescent reagents
- Client provided
- Generated at Aragen.
Table 1: Validated cancer cell lines currently available at Aragen Bioscience
| Tissue Type | Human Cell Line |
| Brain | U87-MG-Luc |
| Breast | MCF-7, MCF-7-Luc, MDA-MB-231, MDA-MB-231-Luc, MDA-MB-468, SK-Br-3 |
| Colorectal | Colo-205, HT-29, HCT-116, HCT-116-Luc, SW480, DLD-1 |
| Epidermoid | A-431 |
| Gastric | MKN-1, MKN-1-Luc* |
| Head and Neck | FaDu |
| Lueukemia/Lymphoma | Daudi, Ramos, Raji, Raji-Luc, My-La*, NALM-6,SUDHL-5, SUDHL-10, Kasumi-1, KG-1, NCI-H- 929, U266, MM.1, RPMI-8226 |
| Liver | SK-Hep-1, HepG2, Hep 3B, HepG2.2.15* |
| Lung | A549, A549-Luc, Calu-6, H226, NCI-H226, H1299, NCI-H292, HCI-H358, NCI-H1944, NCI- H1573 NCI-H23, NCI-H2171, NCI-H1650 |
| Muscle Ewing’s Sarcoma | A673-Luc* |
| Melanoma | A375, SK-MEL-2, Hs-294T, Me-Wo-Rluc* |
| Ovarian | SK-OV-3, SK-OV-3-Luc, Ovcar-3, IGROV-1, OV-90 |
| Pancreatic | BX-PC-3, BX-PC-3-Luc. Mia Paca-Rluc*, Mia-Paca, Panc10.05 |
| Prostate | 22Rv.1, LNCaP, PC3 |
| Renal | 786-0, Caki-1 |
* Cell lines provided by our sponsors
**For new models not previously established at Aragen, we recommend assessing the growth kinetics of in vivo models.
Table 2: Validated cancer cell lines currently available at Aragen Bioscience
| Tissue Type | Human Cell Line |
| Brain | GL-261-Luc |
| Breast | 4T1, 4T1-Luc |
| Colorectal | MC38, MC38-Luc, CT26, CT-26-Luc |
| Lung | LL/2, TC-1* |
| Liver | Hepa 1-6 |
| Leukemia/Lymphoma | A20, EL-4, E.G7, H1210 |
| Melanoma | B16F10 |
| Renal | Renca |
* Cell Lines provides by our sponsors
**For new models not previously established at Aragen, we recommend assessing the growth kinetics of in vivo models.
Syngeneic Models
Methodology
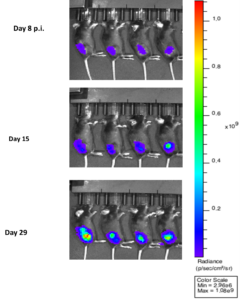
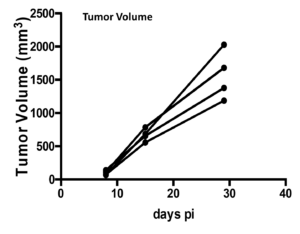
- Implanted 1×106 MC38-Luc cells subcutaneously into the right lower flank of C57BL/6 mice.
- Imaged on day 8, 15 and 29 p.i.
Results
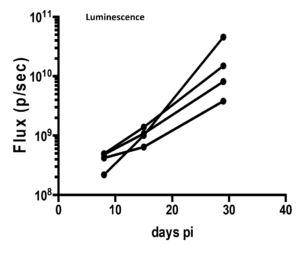
- Maximum tumor volume was attained on Day 29 as shown by the maximum luminescence signals as shown in the following images and graphs.
Note: Aragen has a license for the use of MC-38
Methodology
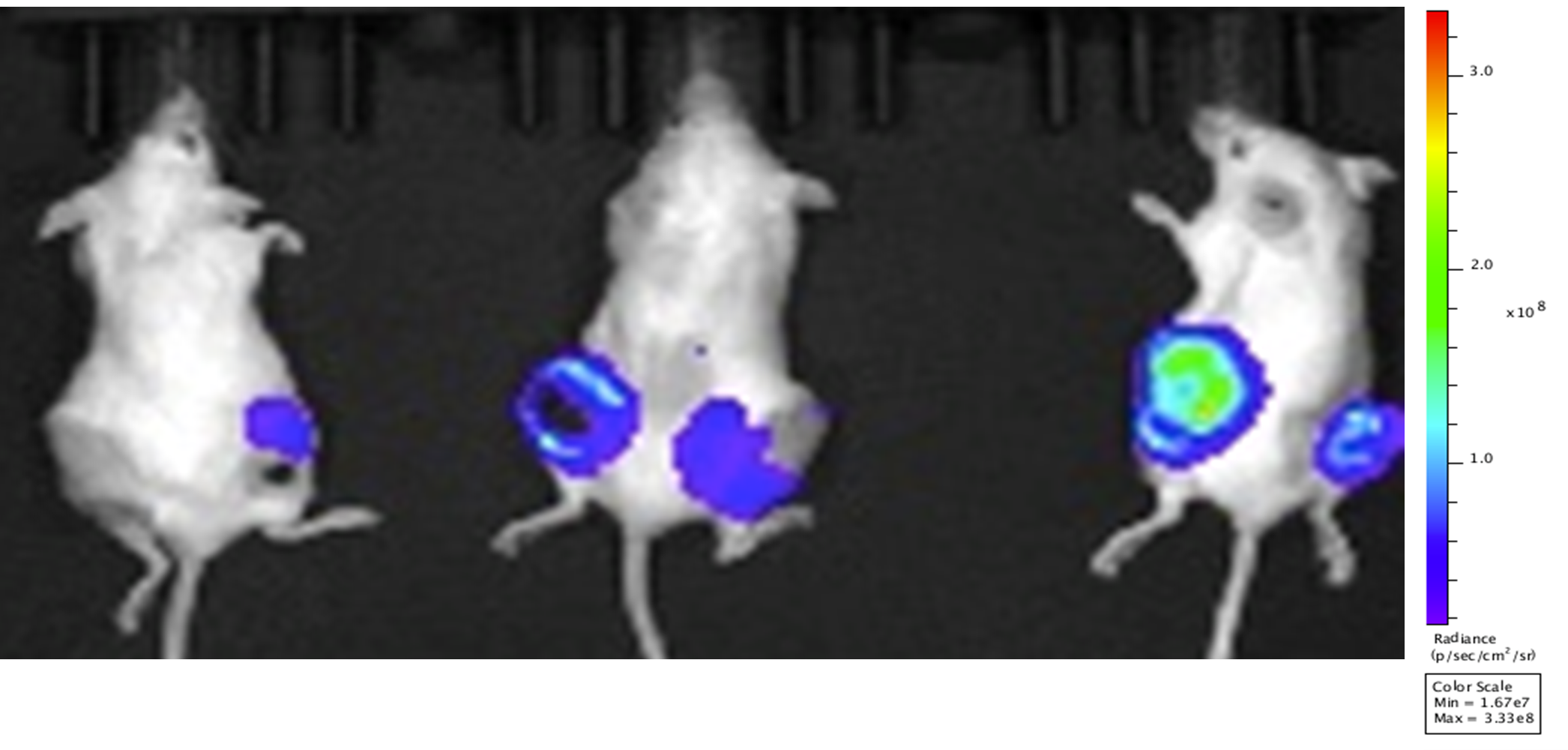
- Implanted xx 4T-1-luc cells subcutaneously into Balb/c mice.
- Mouse Imaged on day 24 p.i.
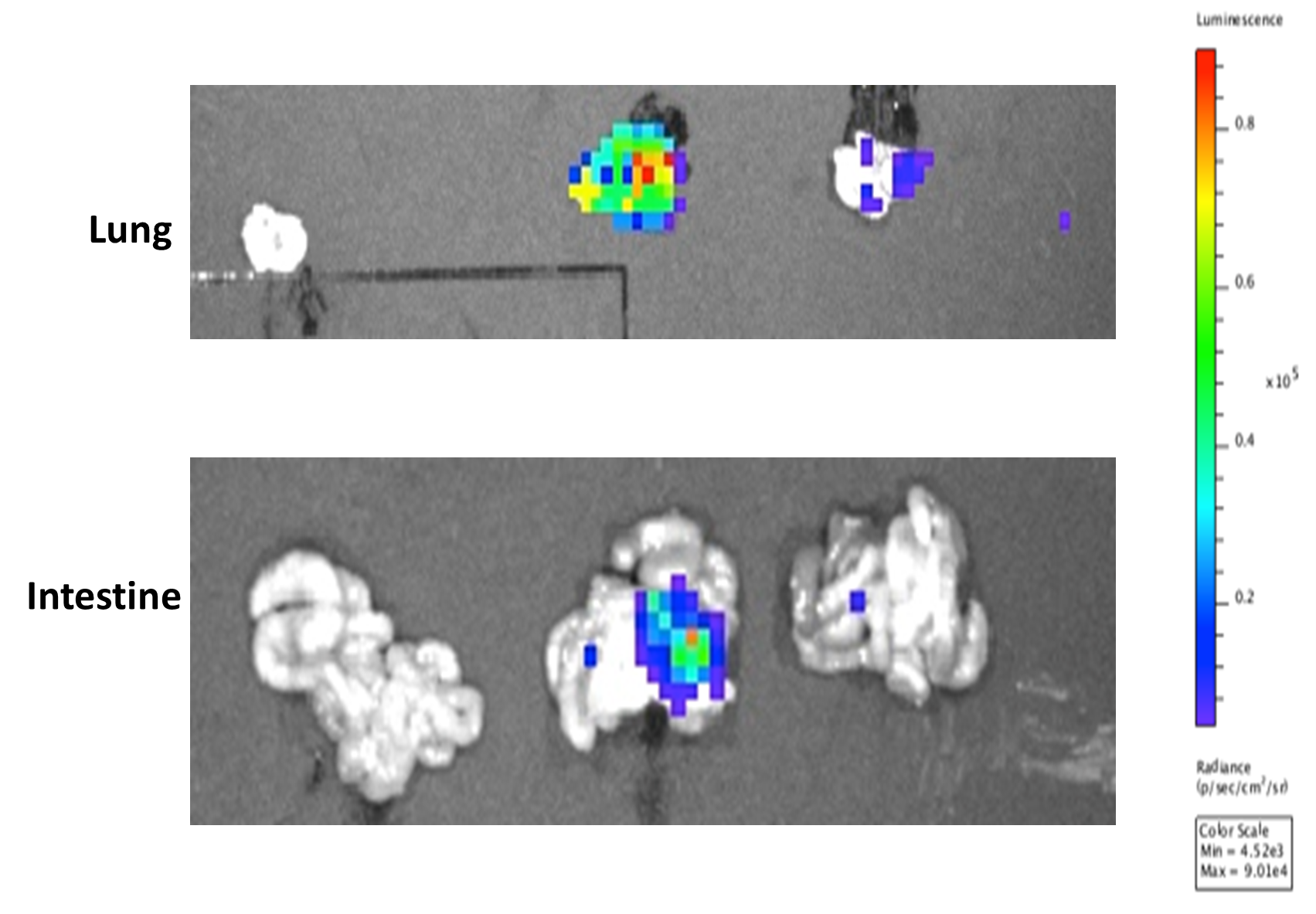
- Study was terminated, lungs and intestine removed and imaged immediately.
Results
- A detectable tumor in the flanks of the mice was attained on Day 24 as shown by the maximum luminescence signal. The luminescence signal is also evident after the ex vivo imaging of the lung and intestine of the mice as shown in the following images.
Xenograft Orthotopic Models
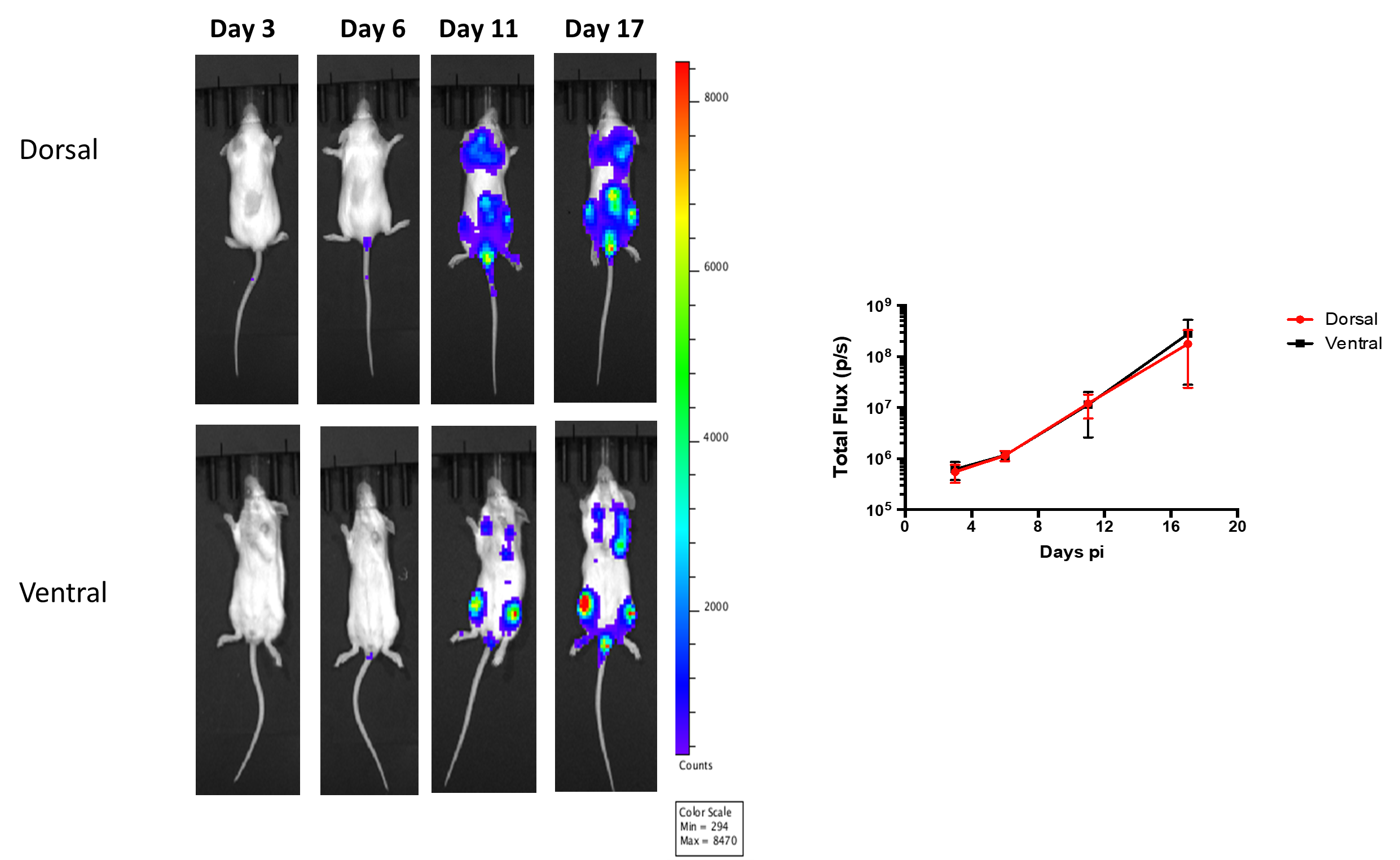
Methodology
- Injected 2×106 Raji-luc cells via tail vein into SCID mice.
- Imaged on days 3, 6, 11, 17, 21 and 25 p.i.
Results
- Dispersed luminescent tumors in dorsal and ventral flanks of the mouse were observed on day 11-17. On Day 17, a maximum luminescence signal was recorded as evident in the following images and graph.
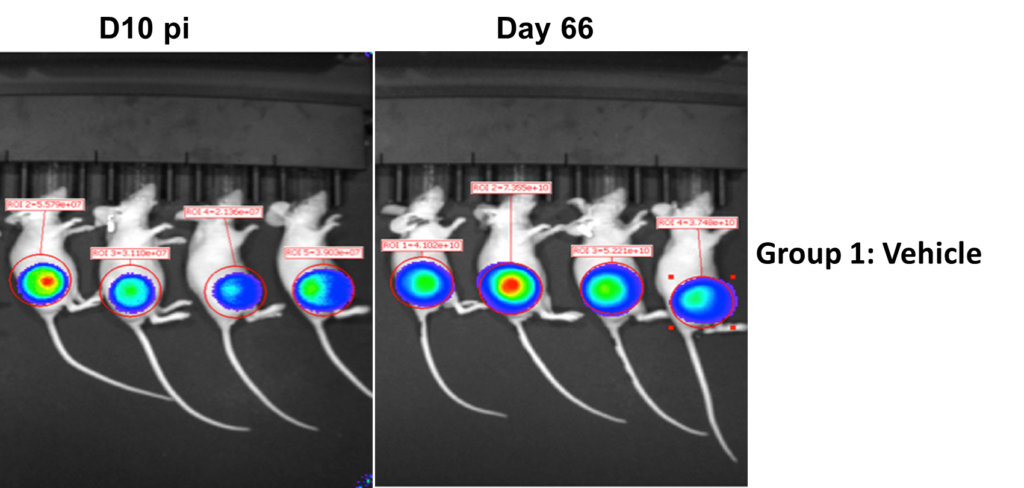
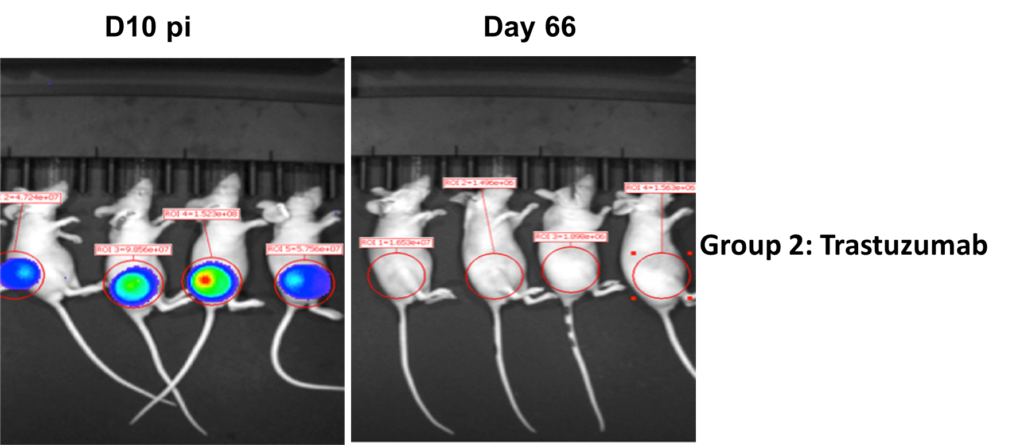
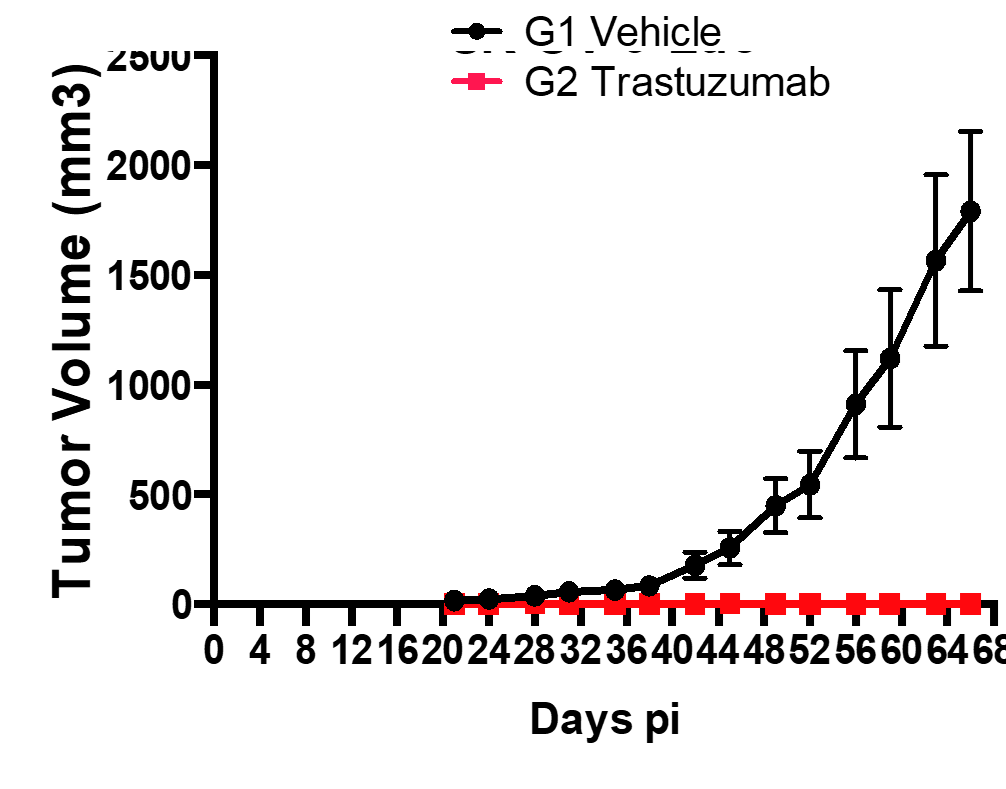
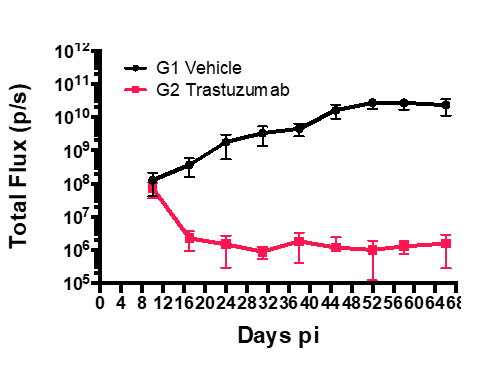
Drug Efficacy in Subcutaneous Tumor Models
Methodology
- Group 1: Vehicle + SK-OV-3-Luc cells
- Group 2: SK-OV-3-Luc cells + Treatment with Trastuzumab
- Injected 2×106 SK-OV-3-Luc cells via tail vein into SCID mice.
- Imaged on days 3, 6, 11, 17, 21 and 25 p.i.
- Trastuzumab treatment was initiated on day 10 p.i.
- Sub cutaneous tumors in the flanks were monitored from day 10-66, both in Group1 and 2.
Results
- Bioluminescent signal enabled detection of early tumor establishment.
- A sub cutaneous tumor in the mouse flank was visible on day 10-66.
- The bioluminescence signal disappeared as the tumor regressed by Day 66 in group 2 after the treatment with Trastuzumab. However, the signal remained strong in the group 2 mice as shown in the following images and graph.
Contact Us to Learn More
Experience the Convenience of ARAGEN:
Based in Morgan Hill, CA, we offer rapid domestic shipping for temperature-sensitive samples and therapeutics. We are excited to collaborate with you, contribute to your research endeavors, and pave the way for your success.
Your research deserves the excellence and precision that only Aragen bioscience can deliver. Contact our team in Morgan Hill, California to learn more about Aragen’s oncology models and capabilities.