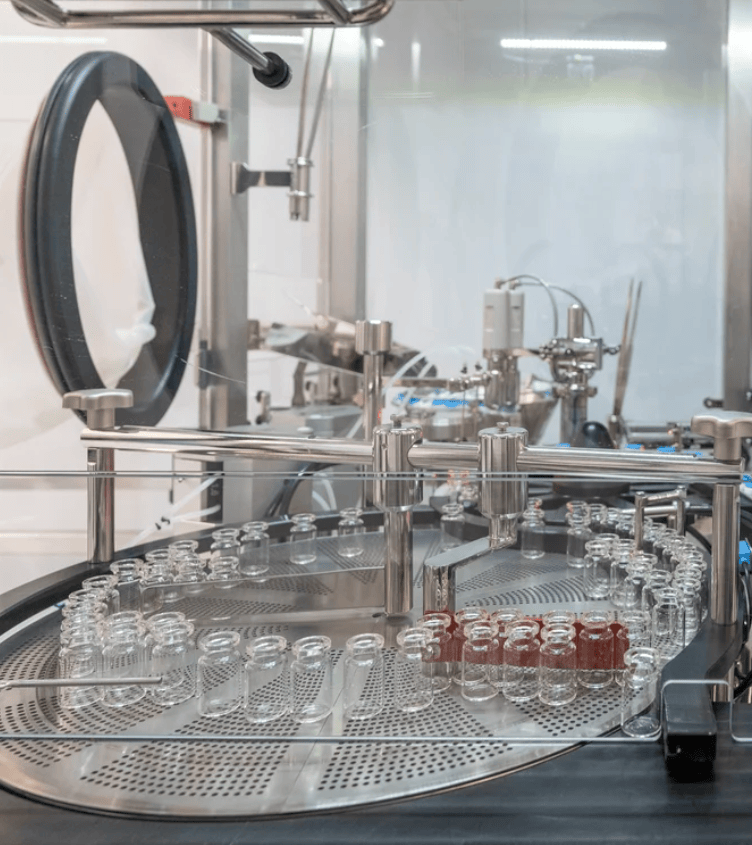
Designed for optimal cost-efficiency and agility, the facility enhances our collaborative approach, providing unmatched flexibility while consistently delivering exceptional quality throughout the entire process. Our manufacturing facility operates in full compliance with GMP standards and all relevant regulatory requirements, demonstrating our commitment to the highest quality in biologics production.
- Innovative facility design
- High-performance biologics development
- Process control & digitization
- Quality control and testing service
- Regulatory support
The Aragen Advantages
- Effective tech transfer: Skilled in transferring later-stage projects and optimizing processes based on insights.
- Predictive scale-up: Predictive models for efficient scale-up from lab to production scale.
- Seamless scalability: Our integrated development and manufacturing model ensures a smooth transition from lab scale (Ambr 250) to pilot scale (50L bioreactor) to production scale (2000L bioreactor) operations.
- Stage flexibility: Supports non-GMP, clinical and commercial stage projects.