Immunogenicity Studies for Vaccine Candidates
Immunogenicity studies are essential for evaluating vaccine candidates, and mice play a crucial role in this research due to their genetic similarity to humans, cost-effectiveness, and well-characterized immune systems. Their genetic makeup can be easily manipulated, allowing targeted studies of immune pathways. In addition, mice are ethically accepted for initial testing, and their controlled environments increase reproducibility. Their small size allows high-throughput screening of multiple vaccine candidates, facilitating efficient evaluation of efficacy, safety, and immune memory development, which is crucial for advancing to clinical trials.
At Aragen, we specialize in conducting comprehensive immunogenicity studies and preclinical vaccine efficacy assessments using in vivo rodent models. Our studies are meticulously designed to evaluate how well vaccine candidates stimulate the immune system to produce protective responses against pathogens. Key components of our immunogenicity assessments include measuring antibody production, T-cell responses, and overall vaccine safety. By gaining insights into these immune responses, we facilitate customers to optimize vaccine formulations and enhance efficacy, ensuring readiness for clinical trials.
Contact us to learn more about our extensive experience running preclinical vaccine studies and the evaluation of the immune responses elicited by the vaccine candidates.
Example Vaccine Design
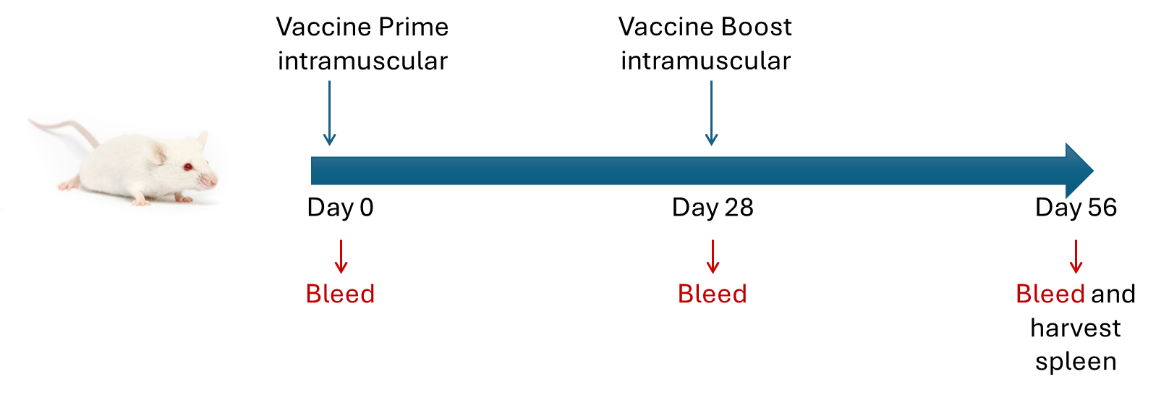
Methodology
- Day 0: Bleed and immunize mice
- Day 28: Bleed and boost mice
- Day 56: Bleed and harvest spleen for analysis
- Monitor weight throughout the study
- Evaluate immune responses in the serum and lymphoid tissue
Depending on the vaccine target, various immune readouts are available which include ELISA, FACS, MDS and ELISpot based assays.