Respiratory Syncytial Virus (RSV) Model
Respiratory Syncytial Virus (RSV) is a common viral pathogen that primarily affects the respiratory tract, particularly in infants, young children, the elderly, and other immunocompromised populations. A member of the Paramyxoviridae family, RSV is a significant cause of respiratory infections, including bronchiolitis and pneumonia.
Aragen offers RSV preclinical efficacy and safety studies using appropriate in vivo rodent models, such as mice and cotton rats, to facilitate the development of anti-RSV antibodies, small molecules, and vaccines. These models are essential for understanding RSV pathogenesis and immune responses, ultimately informing strategies to combat this serious respiratory threat.
|
Virus Strains
|
||||
|
RSVA2
|
RSVA2-line19F
|
RSV B
|
RSV Long
|
Client Provided
|
|
Model Animals
|
||||
|
BALB/c Mouse
Cotton Rat
|
||||
RSV Vaccine Model
At Aragen, we offer an advanced RSV vaccine model that rigorously assesses the efficacy and safety of potential candidates. Our model provides in-depth analyses of immune responses, precise evaluations of viral control, and comprehensive assessments of lung function and histological changes, delivering critical insights into respiratory health.
This model also supports the development of effective therapeutic strategies, ensuring your vaccine is safe and impactful. Utilizing our scientifically validated RSV vaccine model gives you a competitive edge in informed decision-making and accelerates the path to a successful RSV vaccine. Let us help you advance respiratory health solutions.
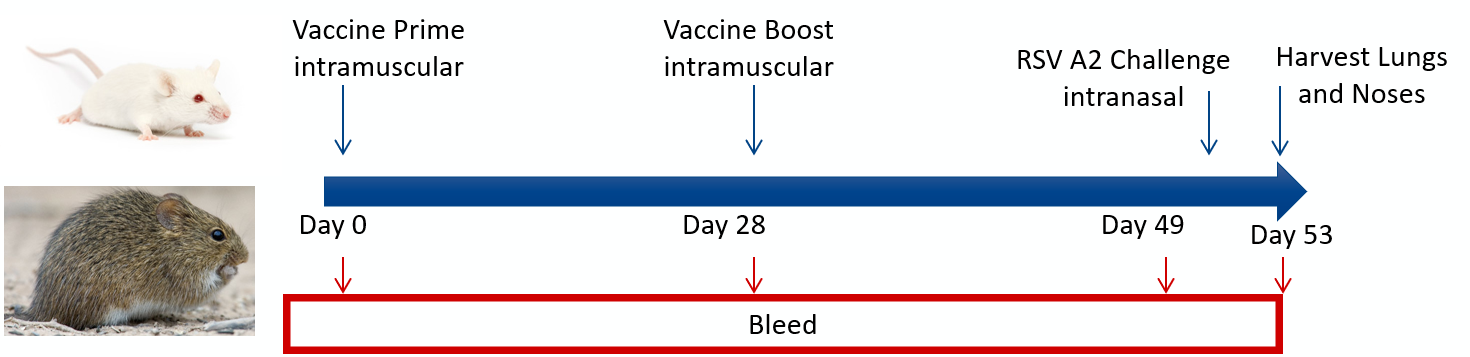
Key Readouts
- Lung and nose viral titers by plaque assay
- Lung and nose viral genome copy numbers by qPCR
- Immune response modulation: Neutralization Assays, ELISA, ELISpot, FACS, MSD
- Bronchoalveolar lavage with cytokine and infiltrating cell analyses
- Lung function readouts: Pulse oximetry, whole body plethysmography, and flexivent
- Histology
RSV Model for Anti-RSV Antibodies and Small Molecules Evaluation
Aragen offers an RSV Model tailored to enable thorough evaluation of anti-RSV antibodies and small molecule therapeutic agents. This innovative model provides researchers with a comprehensive platform to investigate the efficacy and safety of emerging treatments, offering valuable insights into the intricate interactions between the virus and the host immune response. Through rigorous experimentation and advanced methodologies, we strive to drive your progress in RSV research, ultimately contributing to the development of safe and effective therapies.
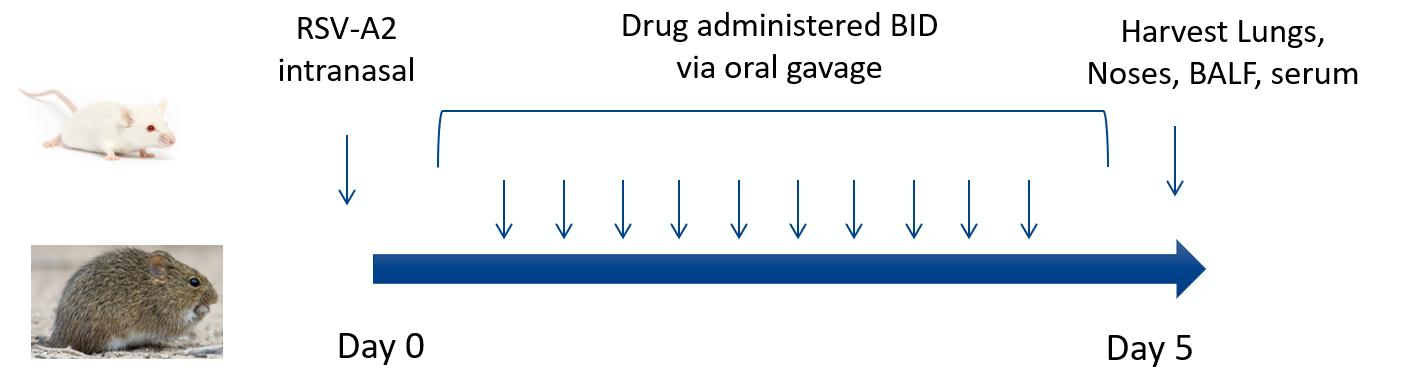
Key readouts
- Lung and nose viral titers by plaque assay
- Lung and nose viral genome copy numbers by qPCR
- Immune response modulation: FACS and MSD
- Lung function readouts: Pulse oximetry, whole body plethysmography, and flexiVent
- PK bleed in-life studies with ELISA serum analysis-blood chemistries
Consistent Bioassay Performance Across Studies with RSV-A2
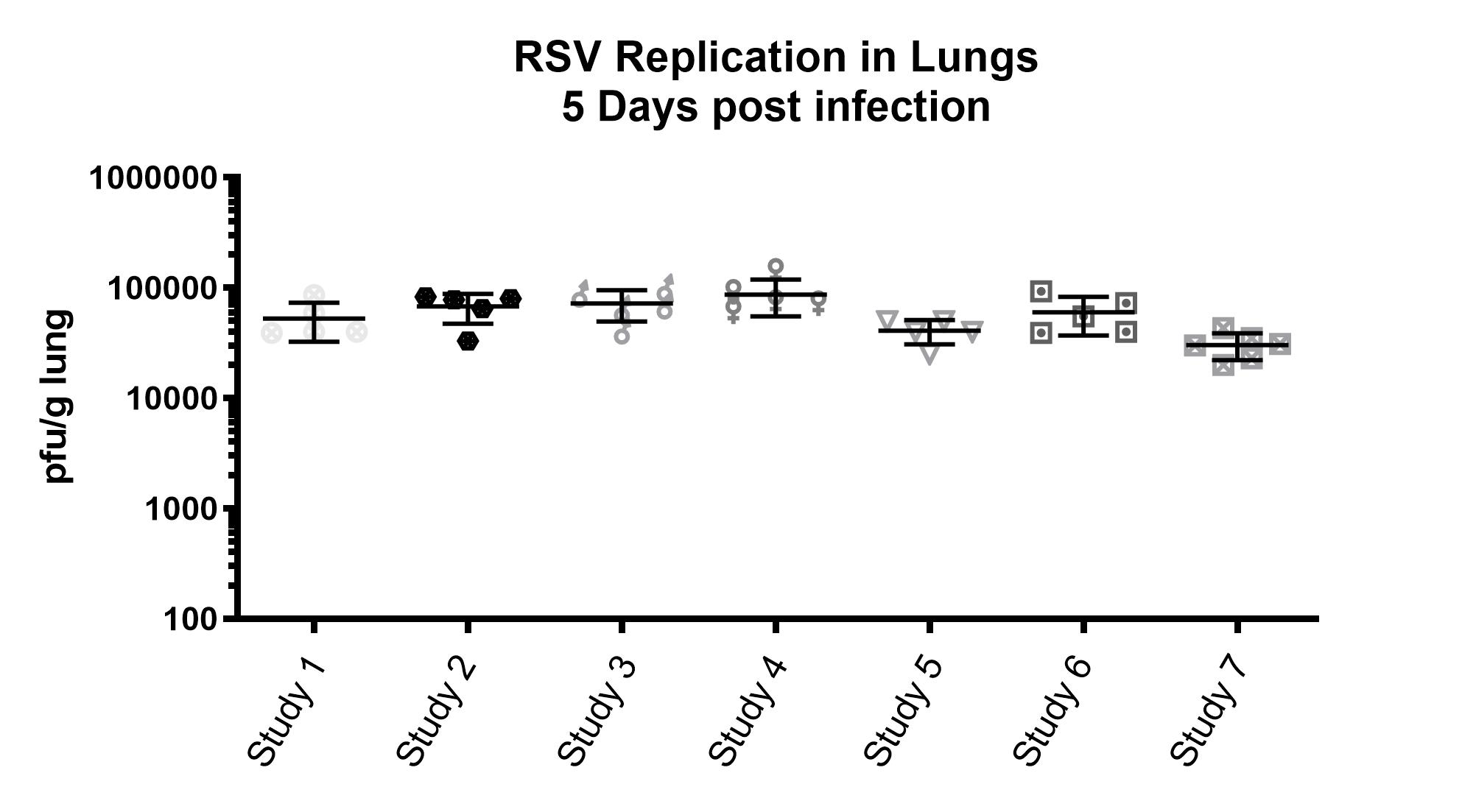
- Female BALB/c: 6-8 weeks of age
- Intranasal infection with RSV-A2
- Large lot of single use aliquots expanded from RSV-A2 ATCC stock (VR 1540)
- Harvest: Day 5
- Lung titers assessed via plaque assay
RSV Model Case Studies
Methodology
- Day 0: Intranasally infected female BALB/c mice 6-8 weeks of age with desired dose of RSV-A2
- Treatment groups: Mock, RSV-A2 + Vehicle, RSV-A2 + Ribavirin as a positive control
- Harvest day 5 post-infection: Collect the lungs and BALF, used for titration by plaque assay and MSD cytokine/chemokine analysis and cellular infiltration analyses
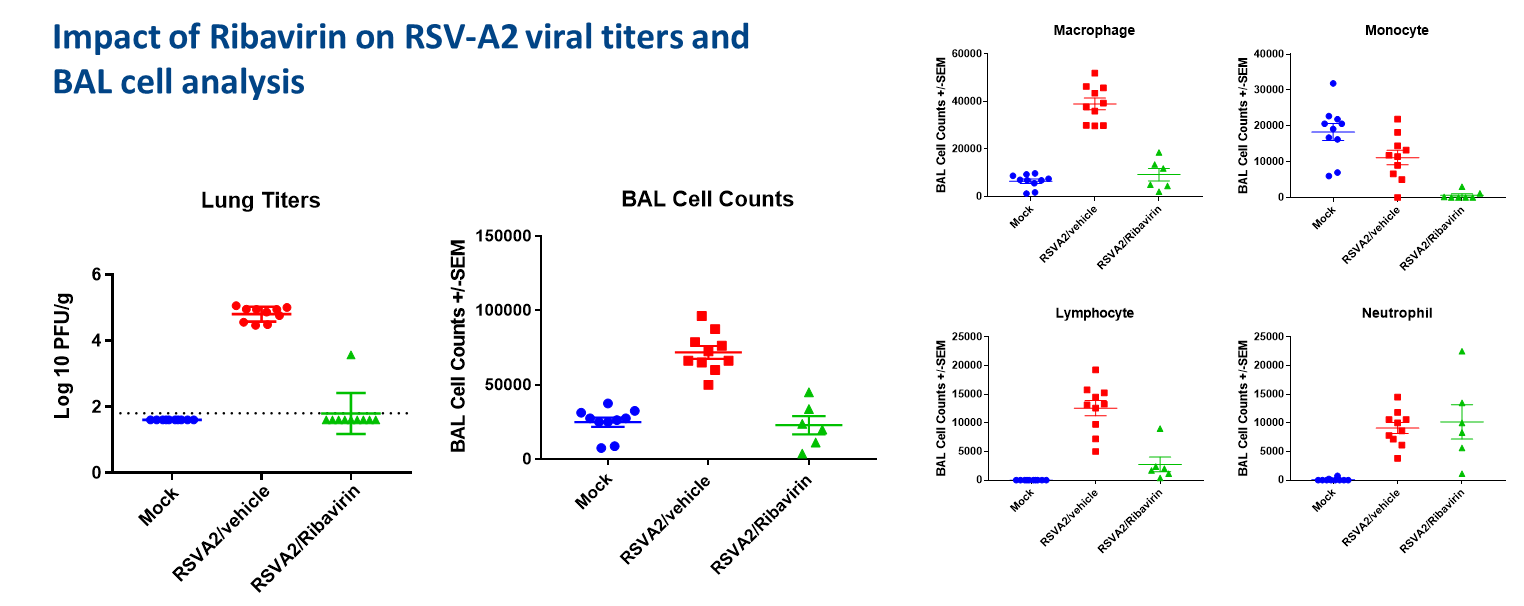
Results
- Ribavirin treatment reduced the RSV-A2 titers in lung
- Ribavirin reduced BAL cell counts including macrophages, monocytes, lymphocyte and neutrophils
- Ribavirin diminished cytokines/chemokines in BAL fluids

Methodology
- Day 0: Intranasally infected female Cotton Rats: 6-8 weeks of age with RSVA2 (ATCC VR-1540)
- Female Cotton rats: 6-8 weeks old
- RSV strain: RSV-A2 (ATCCVR-1540)
- Route of infection: Intranasal
- Clinical observations: Daily
- Harvest on day 4, 5, and 6, post-infection: Collect lungs and noses for titration by plaque assay
- Harvest lungs and noses: Day 4, 5 or 6
- Viral plaque assay on homogenized tissue
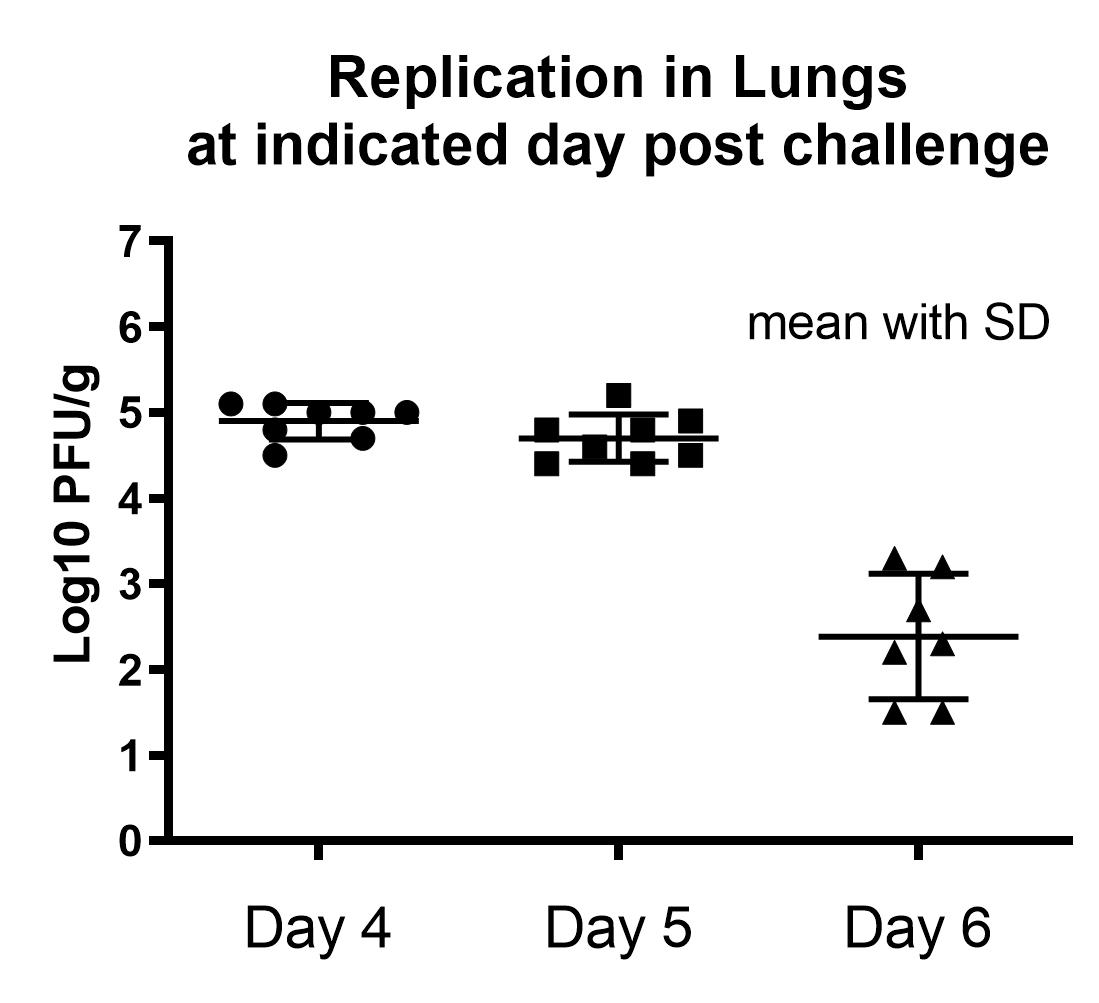
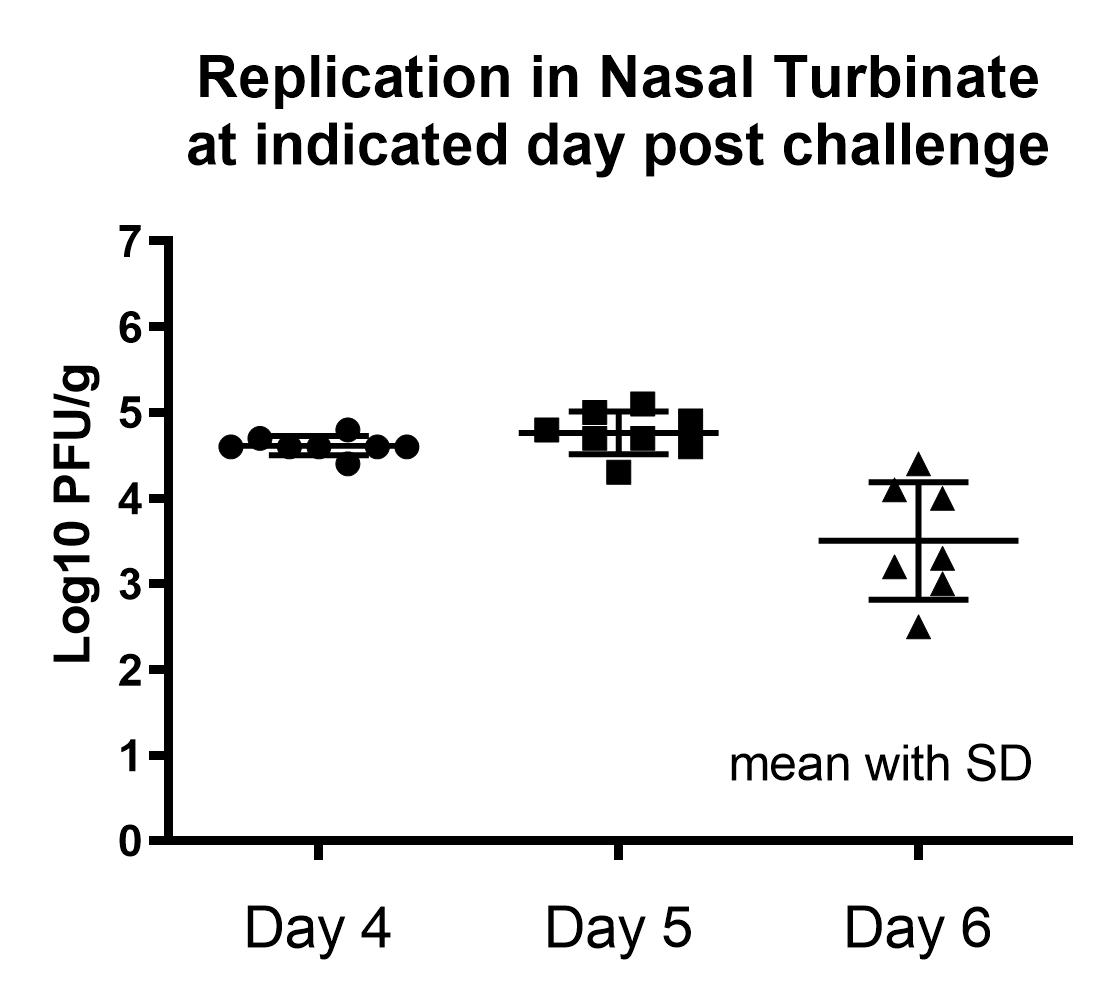
Result
- Peak RSV replication observed in 4-5 days post-challenge in both lung and nasal turbinate.
Mouse PR8 Model for Influenza
Influenza poses a significant public health threat, with seasonal outbreaks and pandemic potential resulting in high morbidity and mortality. Aragen’s Mouse PR8 Model for Influenza provides an advanced preclinical platform to enhance your research efforts. This model offers access to a highly virulent strain, PR8, short for A/Puerto Rico/8/1934 (H1N1), a strain of the influenza virus that has been adapted for use in mice that simulates lethal influenza infections, allowing for precise studies of the disease progression and host responses.
Designed for comprehensive investigations, our model enables detailed analysis of both innate and adaptive immune responses, including immune cell interactions, cytokine production, and antibody responses—essential for unraveling the complexities of the disease. By closely mimicking key aspects of human influenza, such as weight loss, lung pathology, and lethality, our model provides measurable endpoints for assessing disease severity and treatment outcomes.
At Aragen, our experienced team excels in evaluating the efficacy of vaccines and antiviral drugs, helping you assess their protective effects and therapeutic potential against the influenza virus. Partnering with us will accelerate your influenza research efforts and empower you to make significant contributions to public health strategies.
Methodology
- Day 0: Infect intranasally with desired dose
- Monitor weight and survival throughout the study
- Day of harvest: Lung weights, BAL cell counts, lung titers, and lung histology
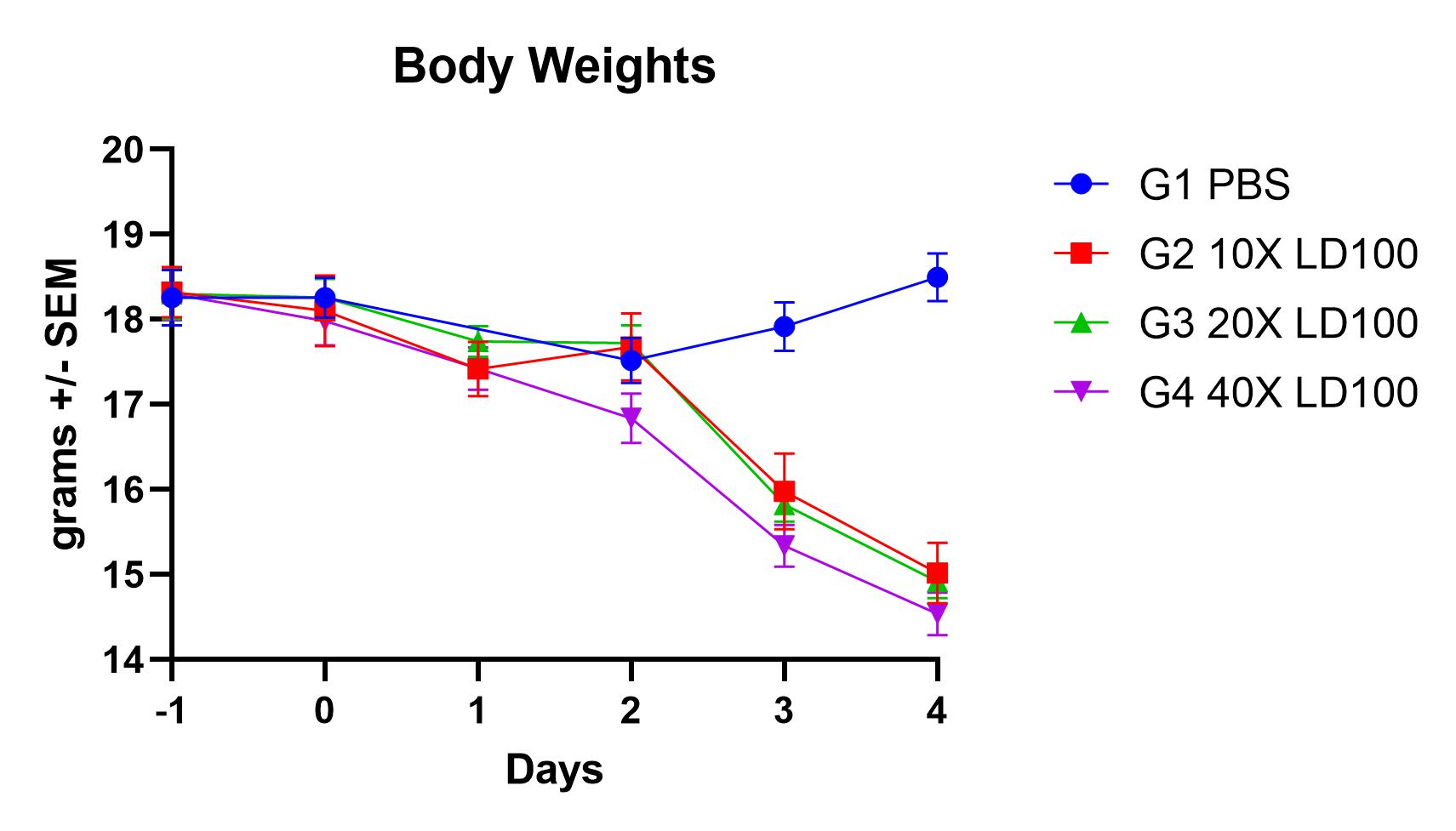
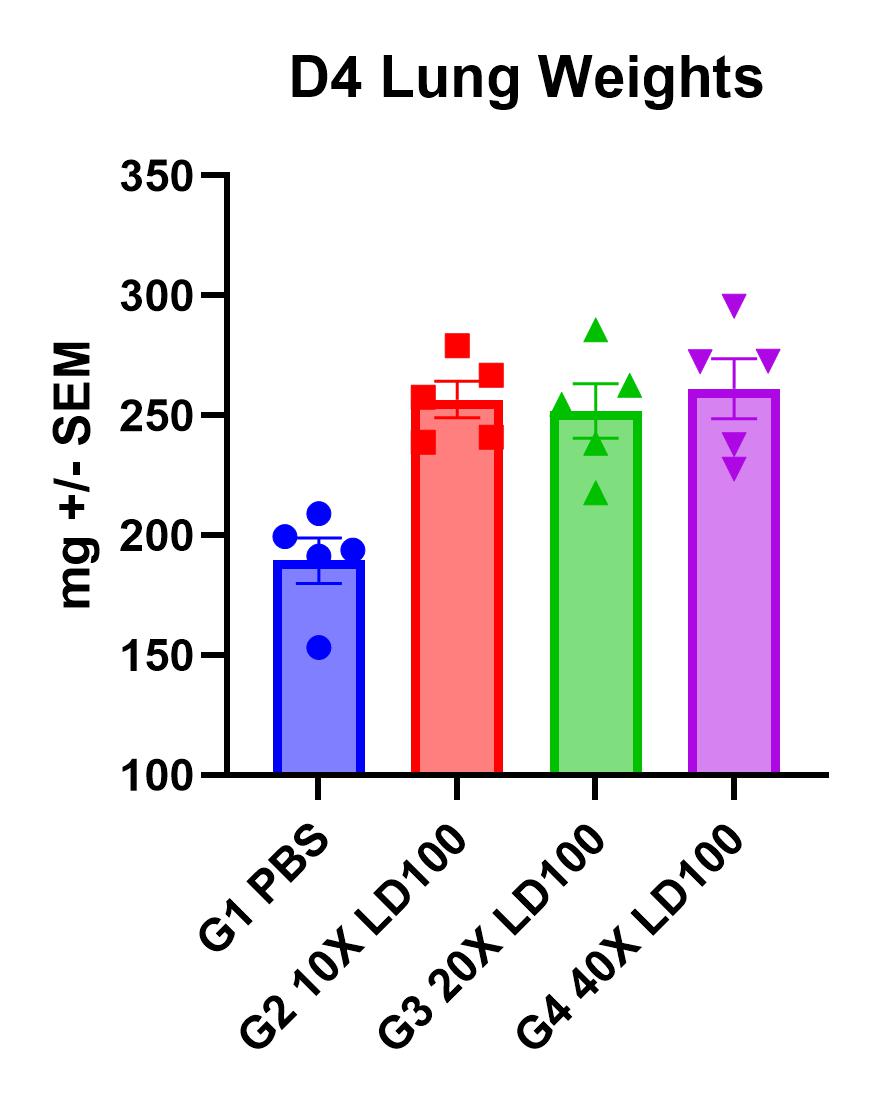
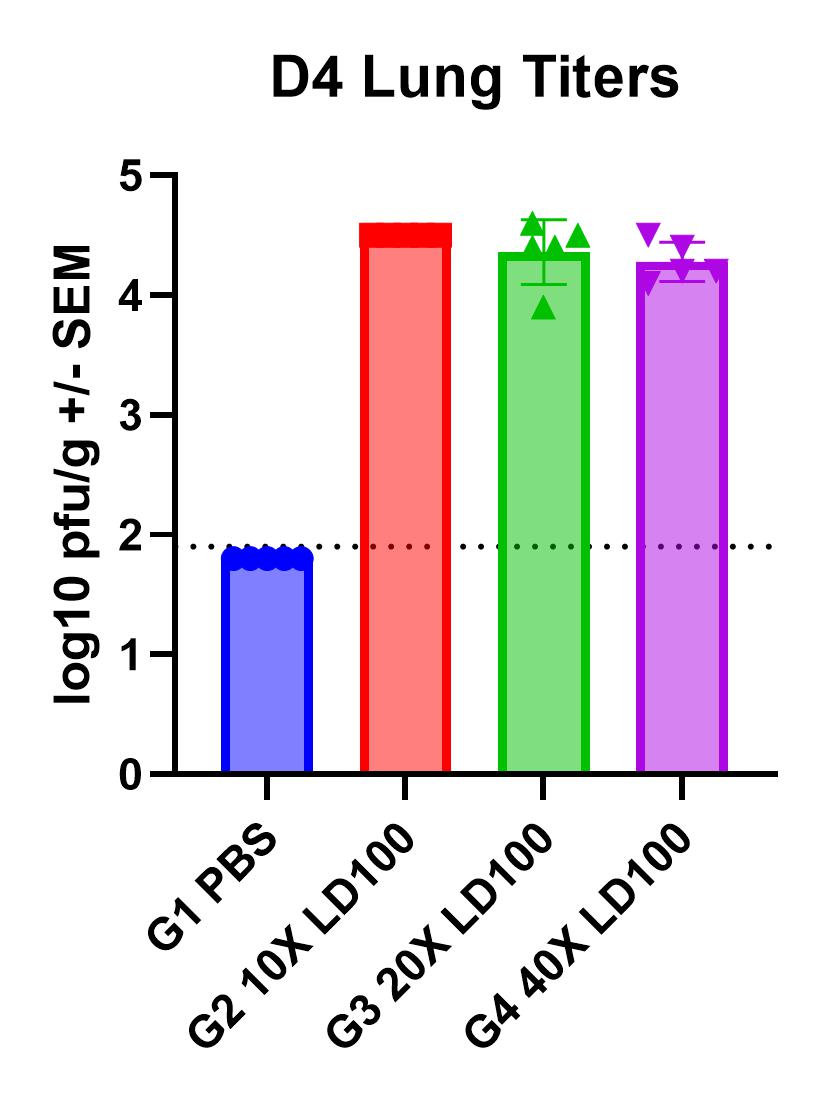
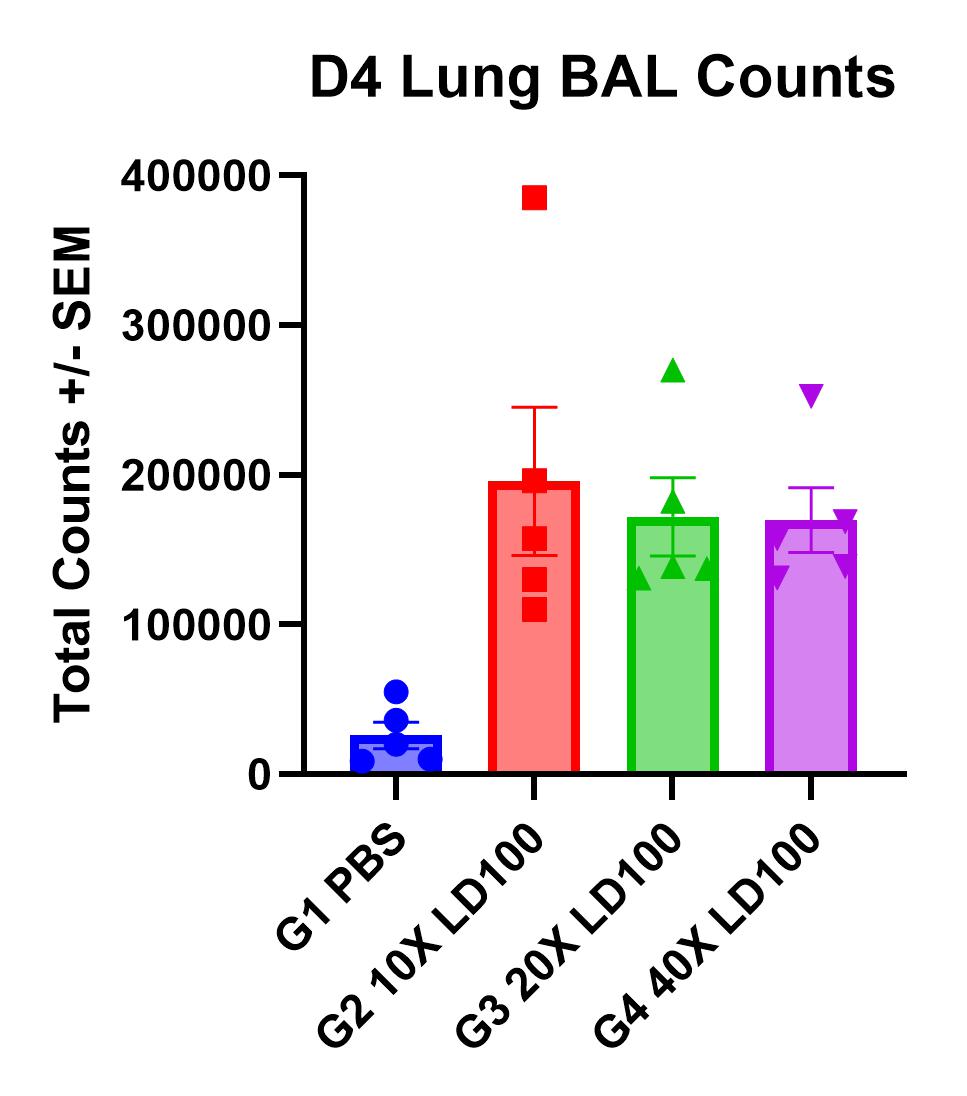
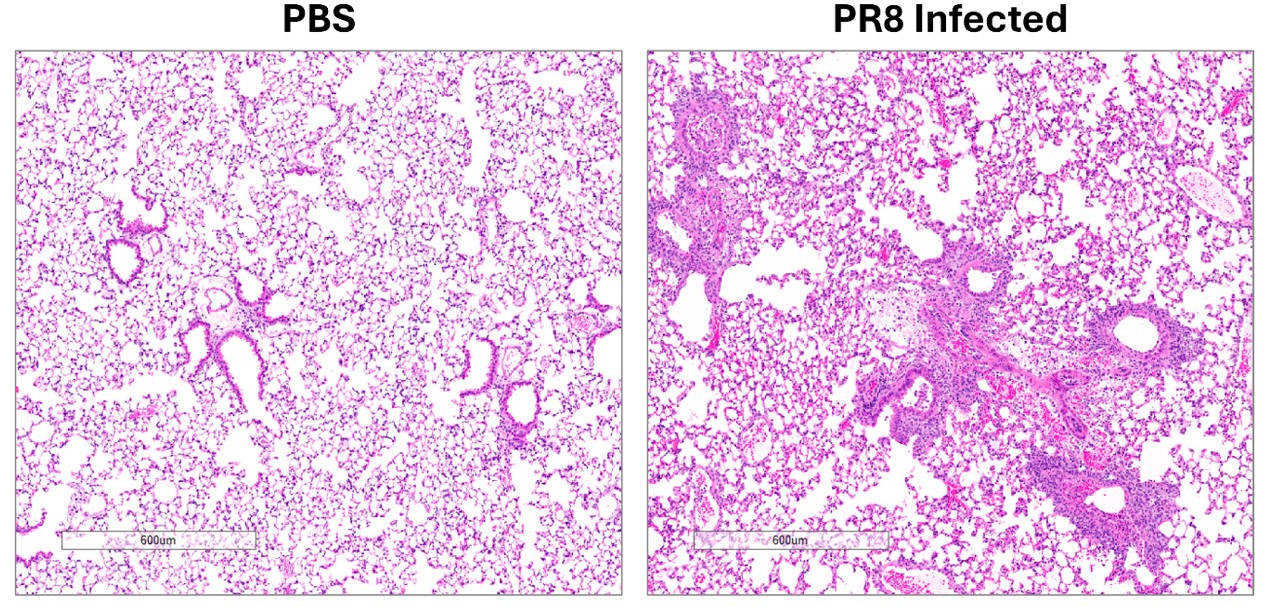
Results
- Viral replication detected in lungs
- Infection manifests as body weight loss, an increase in lung weights, and elevated BAL cell counts
- Inflammatory response assessed by H&E
Methodology
- Day 0: infect intranasally with desired dose
- Monitor whole-body plethysmography and pulse oximetry performed at definite time points throughout the study
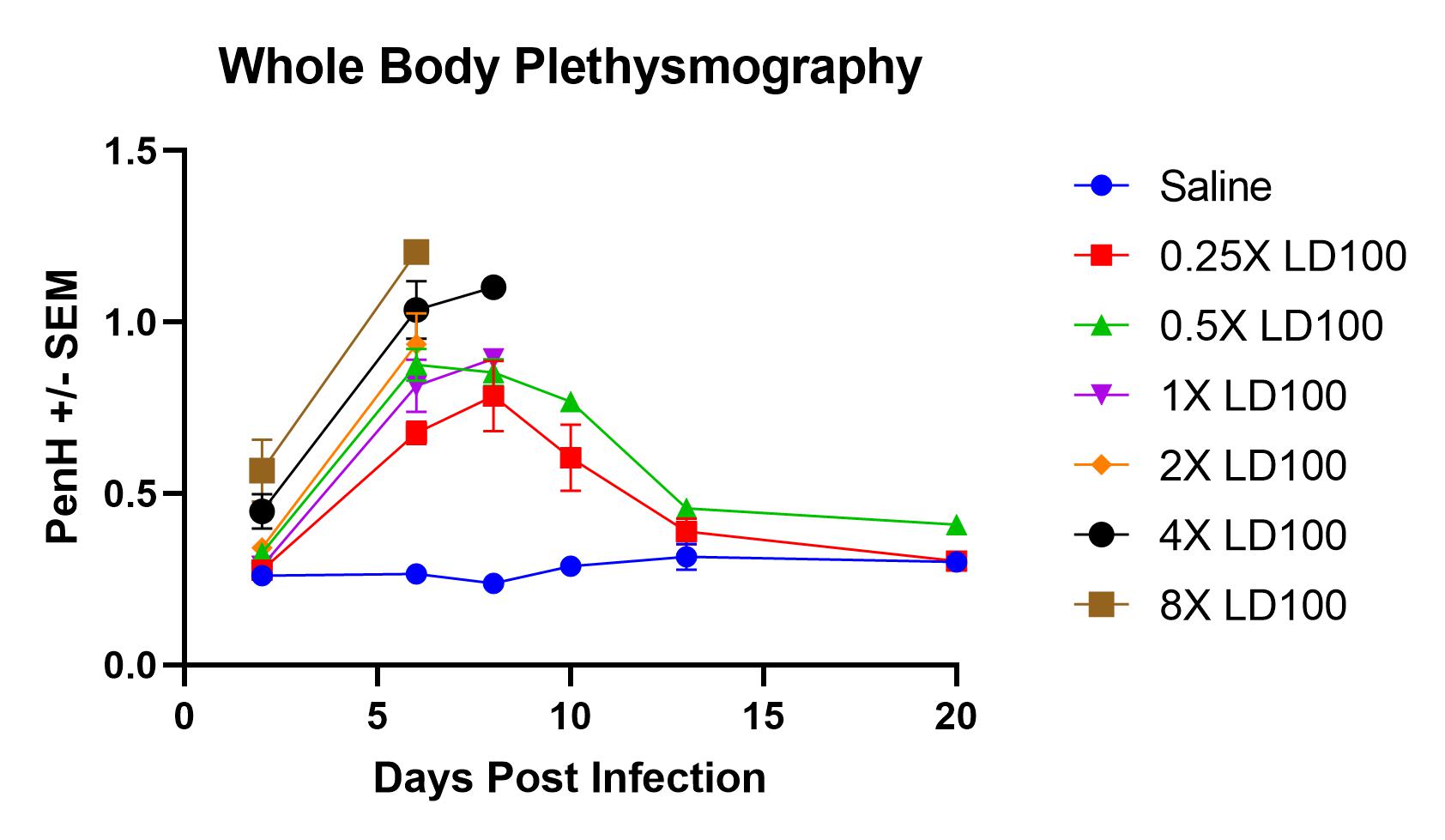
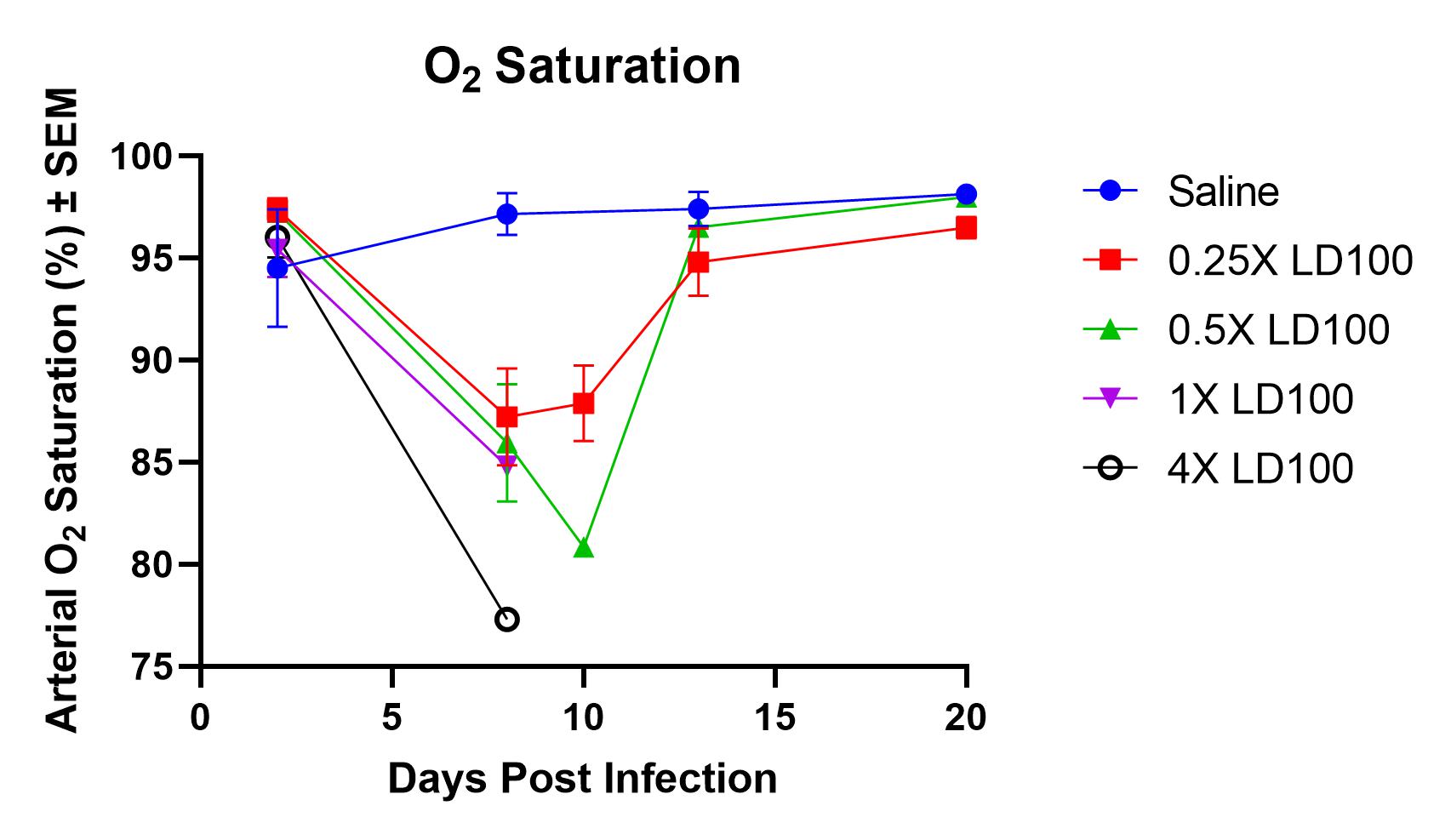
Result
- Infection with PR8 results in an increase in PenH (enhanced pause) levels and a decrease in O2 saturation levels.
MHV (Mouse Hepatitis Virus)-A59 Model for Coronavirus
MHV (Mouse Hepatitis Virus)-A59 is a coronavirus related to SARS-CoV and SARS-CoV-2. Since SARS-CoV-2 does not bind to the murine ACE2 receptor, rodent studies with this virus are restricted to transgenic animals. In contrast, MHV-A59 binds to the murine CEACAM-1a receptor, making it an effective surrogate model for studying coronavirus infections that can be performed in a BSL-2 facility. When dosed via a respiratory route, MHV-A59 induces inflammatory leukocyte infiltrations in the lungs, hemorrhages, and fibrosis of alveolar walls (Yang, 2014). The pathogenesis, including tropism and virulence, shows similarities to SARS-CoV-2, providing a valuable platform for understanding coronavirus-related disease mechanisms.
MHV-A59 serves as an excellent model for studying the pathogenesis, tropism, virulence, and immune response to coronaviruses. At Aragen, by leveraging the MHV-A59 model, we facilitate customers to unlock vital insights into viral pathogenesis, tropism, virulence, and immune response to coronaviruses. Our study design enables thorough evaluation of antiviral candidates, ensuring reliable results for drug and vaccine testing before progressing to human trials. By partnering with Aragen, expedite your research and development processes, enhance the quality of your findings, and position yourself at the forefront of antiviral innovation. Contact us to learn more about our capabilities with the MHV-A59 model.
Methodology
- Day 0: Infect intranasally with desired dose
- Monitor weight and survival throughout the study
- Day of harvest: Lung weights, BAL cell counts, lung titers, lung cytokines and lung histology
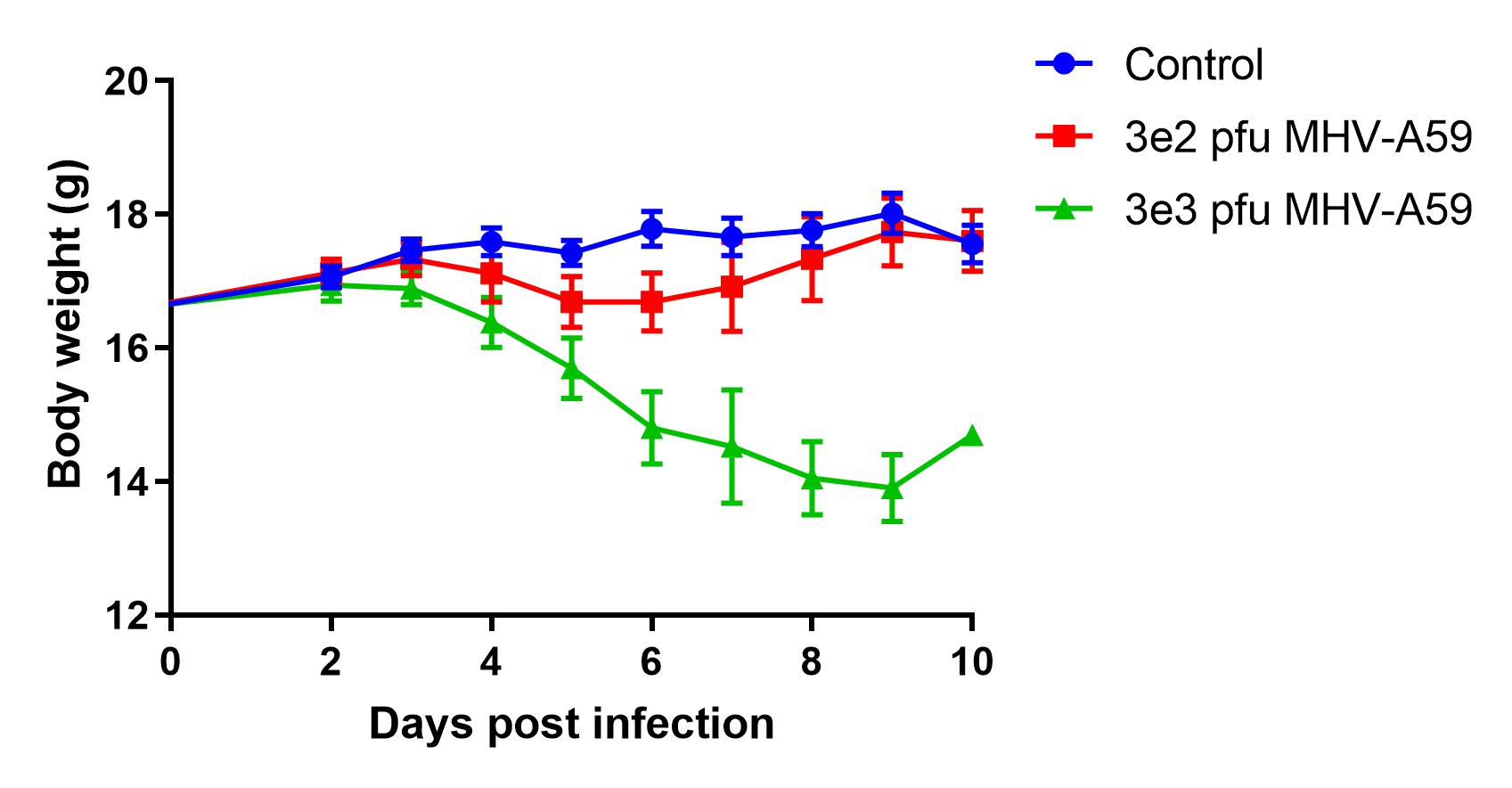
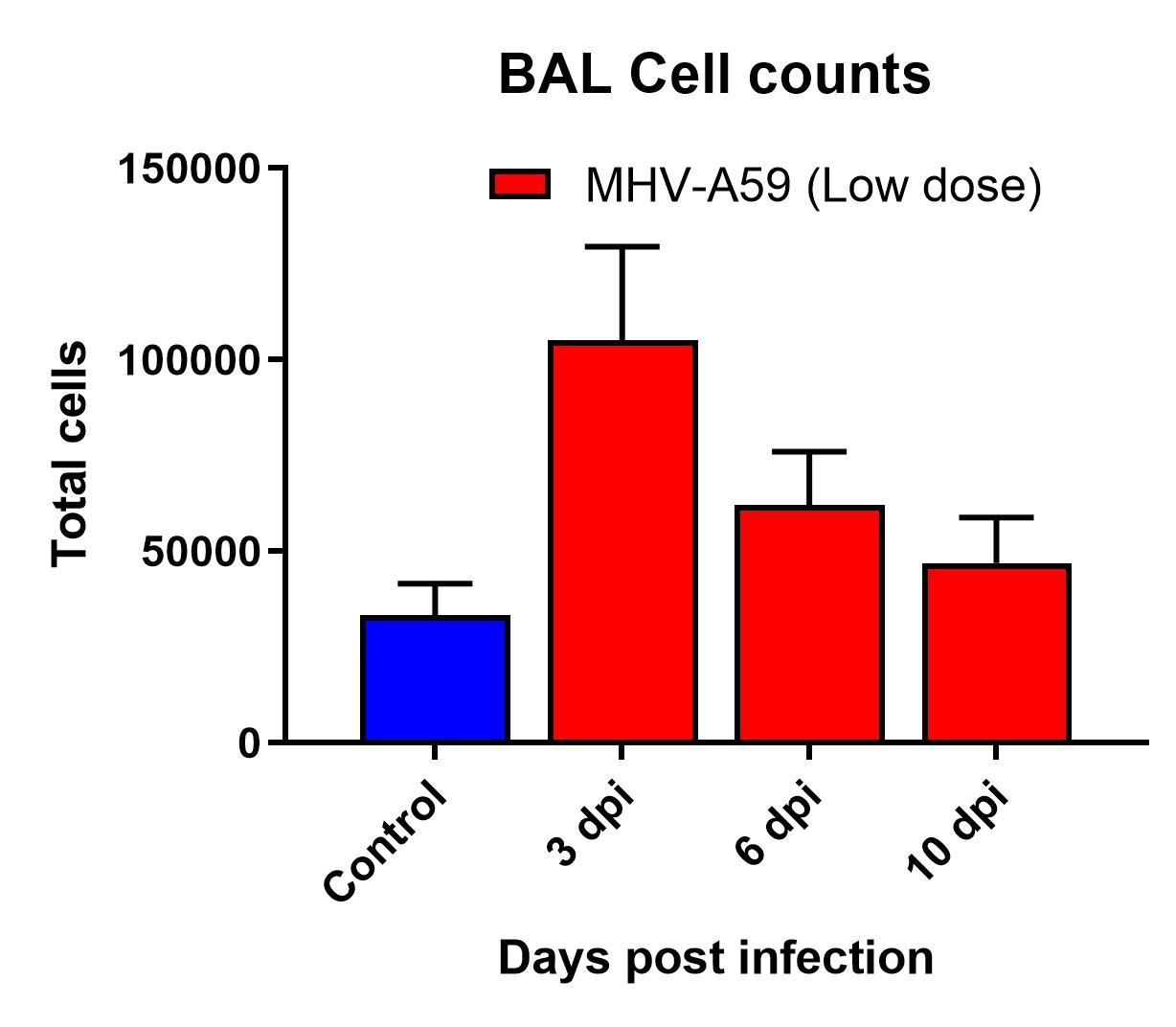
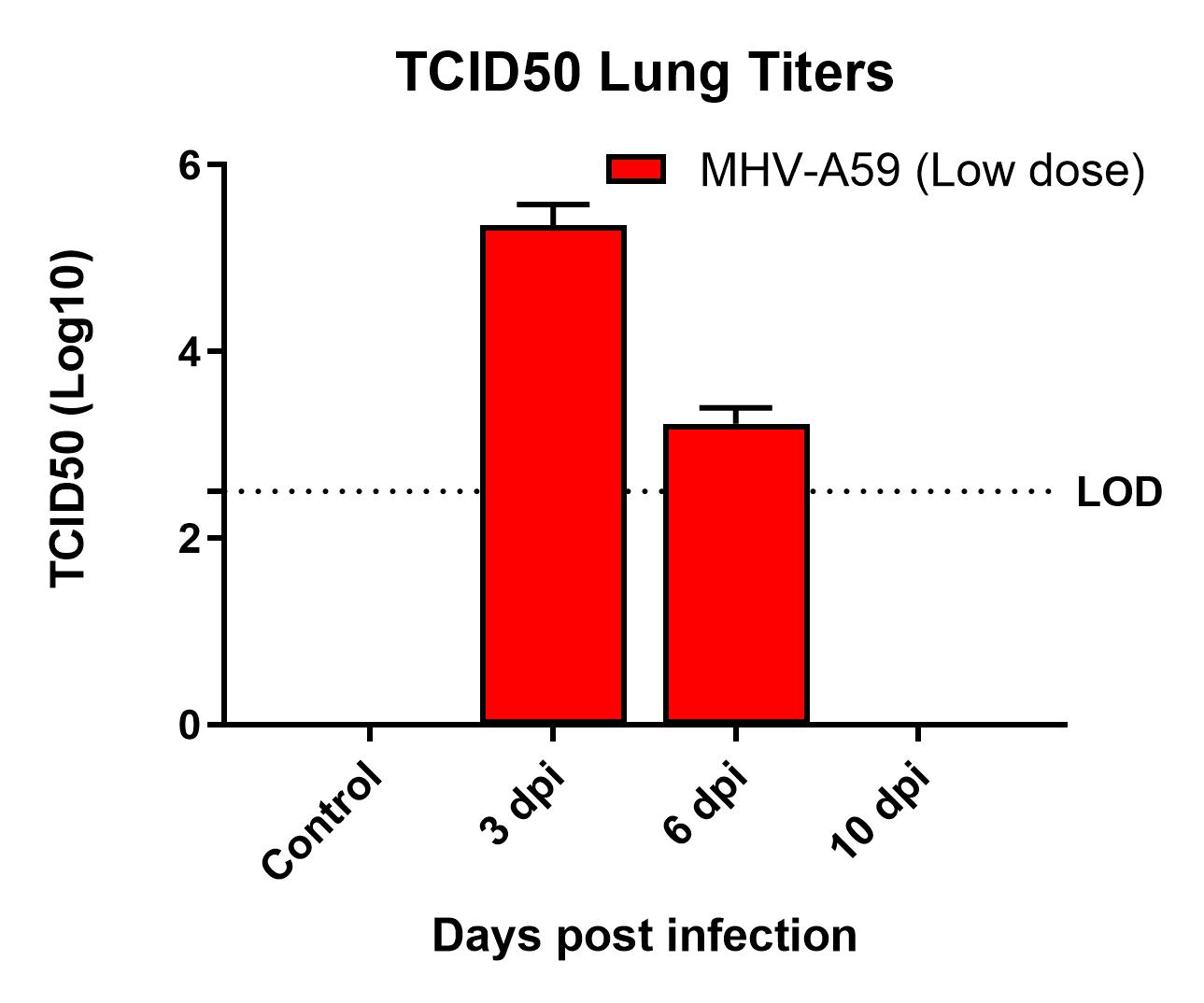
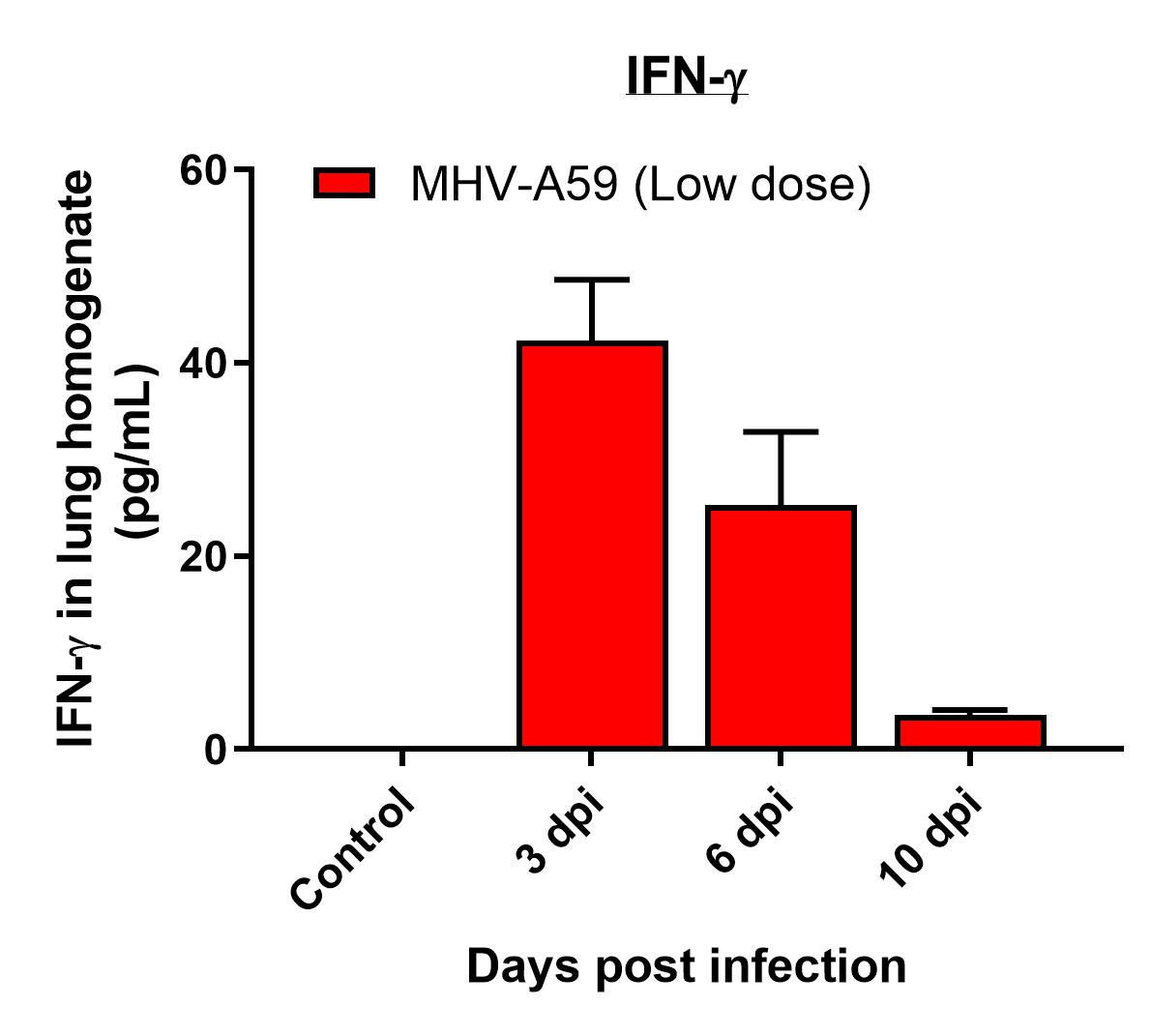

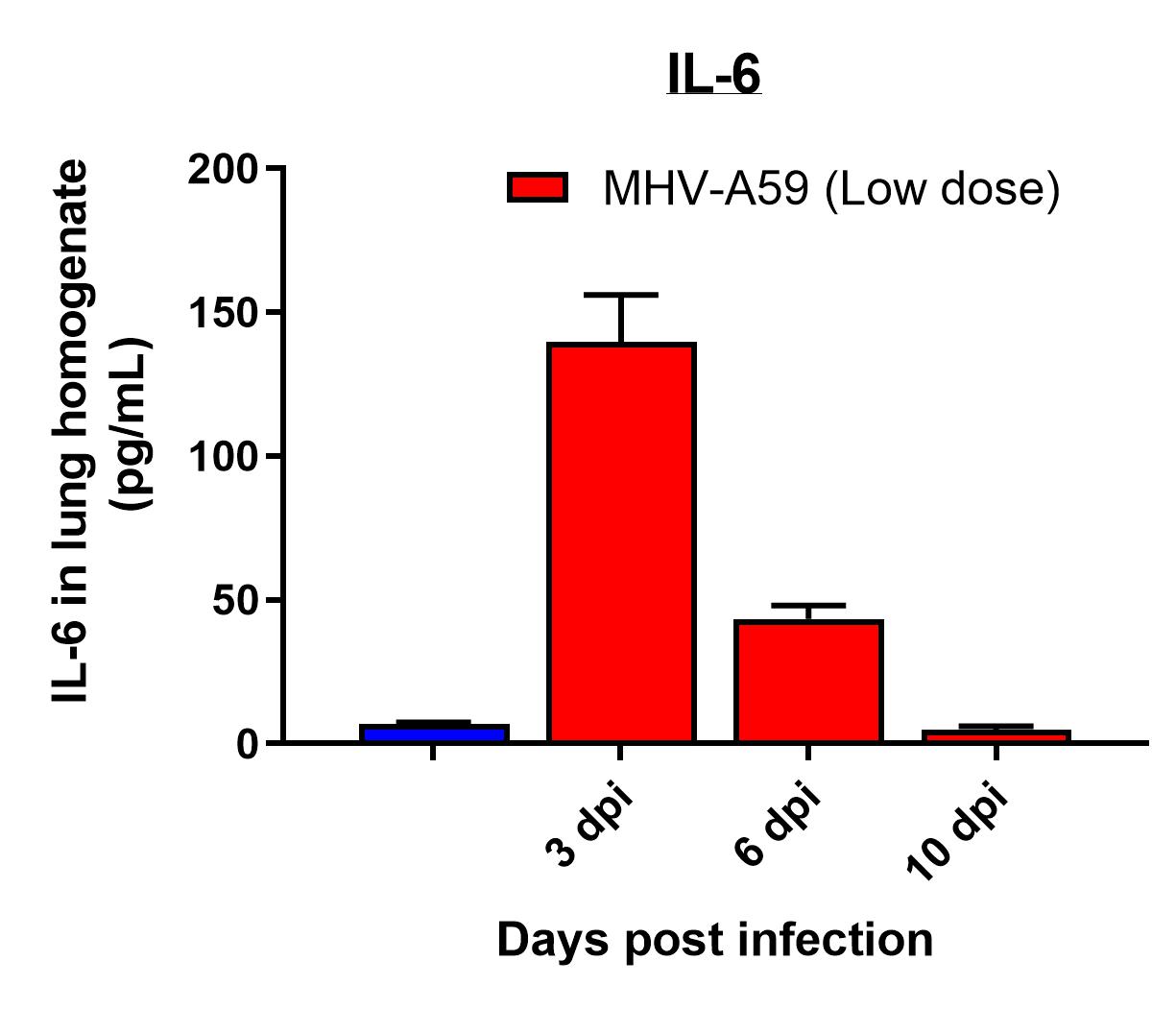
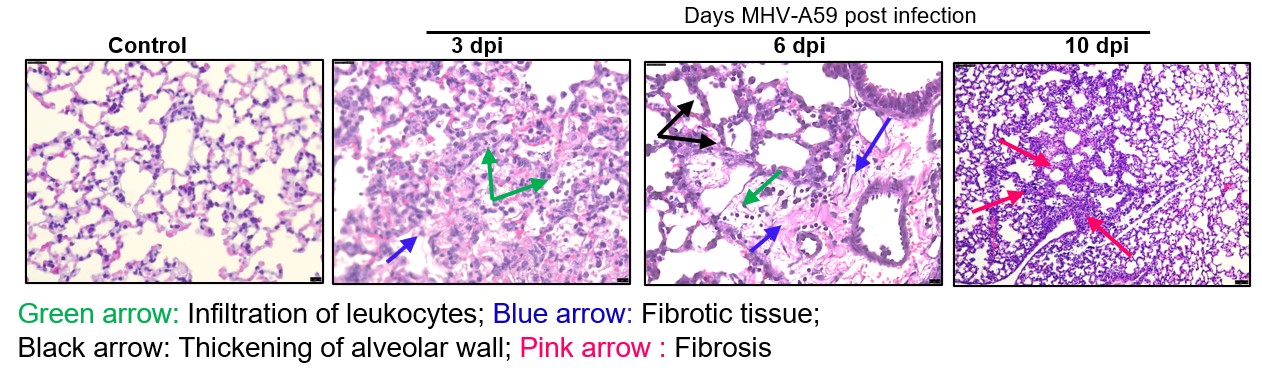
Results
- Viral replication detected in lungs
- Infection manifested as body weight loss, increased BAL cell counts, and elevated inflammatory cytokines levels
- Inflammatory response observed by H&E staining