Aragen’s Infectious Disease Models
Appropriate in vivo rodent models are critical to evaluate the safety and efficacy of new vaccine and antiviral therapeutics. With support for a range of infectious disease models, along with a diverse portfolio of ex vivo assays, Aragen is ideally suited to evaluate discovery and pre-clinical candidates. Our facility in Morgan Hill, California includes a state-of-the-art BSL-2 vivarium.
Capabilities include:
- Immune response readouts such as neutralization assays, ELISA, ELISpots, FACS
- Whole Body Plethysmography, flexiVent, and Pulse Oximetry are available for respiratory disease pathogens
- In vivo and ex vivo readouts
- Viral titrations
- Histology
Respiratory Syncytial Virus (RSV) in Mice and Cotton Rats
Respiratory syncytial virus (RSV) is a common viral pathogen that primarily affects the respiratory tract, particularly in infants, young children, and the elderly. RSV is a member of the Paramyxoviridae family and is a significant cause of respiratory tract infections, including bronchiolitis and pneumonia. Aragen offers RSV preclinical efficacy and safety studies using appropriate in vivo rodent models to enable development of anti-RSV antibodies, small molecules, and vaccines.
| BALB/c Mouse | Cotton Rat | Virus Strains |
|---|---|---|
|
|
|
Experimental RSV Vaccine Model
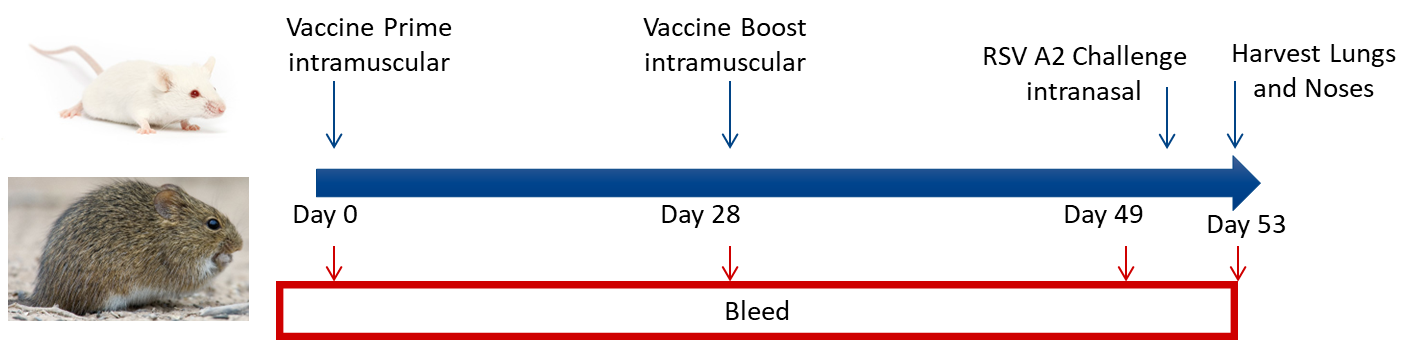
Key Readouts:
- Lung and nose viral titers by plaque assay
- Lung and nose viral genome copy numbers by qPCR.
- Immune modulation: Neutralization Assays, ELISA, ELISpot, FACS, MSD
- Bronchoalveolar lavage with cytokine and infiltrating cell analyses
- Lung function readouts: Whole Body Plethysmography and flexiVent.
- Histology
Experimental RSV Model for Evaluation of Anti-RSV Antibodies and Small Molecules
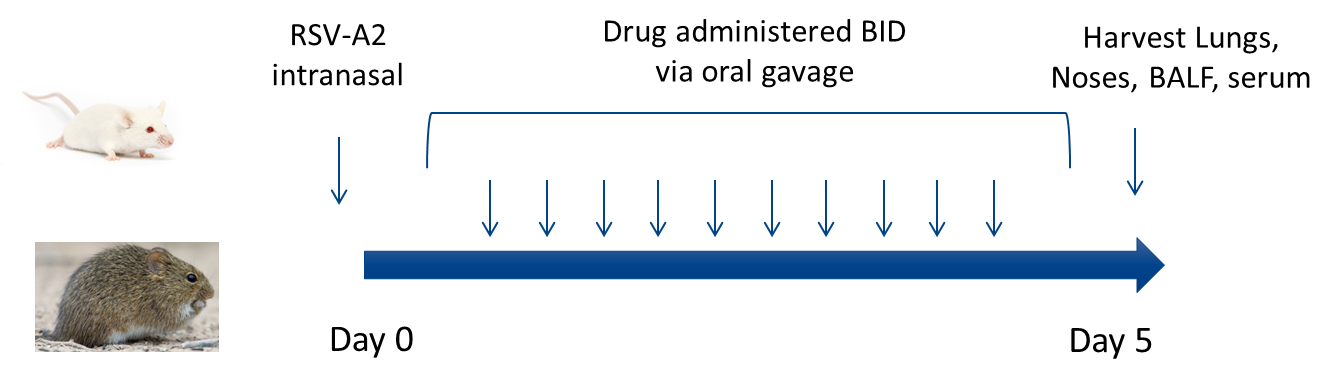
Key Readouts:
- Lung and nose viral titers by plaque assay
- Lung and nose viral genome copy numbers by qPCR
- Modulation of Immune responses monitored FACS and MSD
- PK bleed in-life studies
- ELISA serum analysis-blood chemistries
RSV Model Case Studies
Methodology
- Female BALB/c: 6 weeks of age
- RSV Strain: RSV Long (ATCC: VR-26)
- Inoclulum: Intranasal
- Treatment: 3 Days
- Clinical Observation: Daily
- Tissue Harvest: Day 5
Result
SYNAGIS reduced RSV replication in the lungs of treated mice.
Methodology
- Female BALB/c: 6-8 weeks of age
- RSV Strain: RSV Long (ATCC: VR-26)
- Inoclulum: Intranasal RSV-A2
- Expanded form RSV-A2 ATCC stock (VR 1540)
- Treatment: 3 Days
- Harvest: Day 5
- Bioburden evaluated
- Consistent Bioassay performed across studies
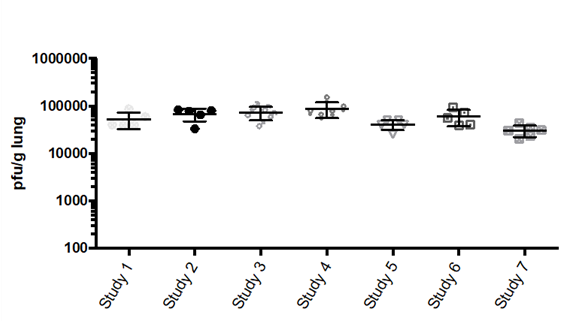
Result
The graph below shows the RSV replication in lungs 5 days post injection across 7 studies.
Methodology
- Female BALB/c: 6-8 weeks of age
- RSV Strain: RSV -A2
- Inoclulum: Intranasal RSV-A2
- Expanded form RSV-A2 ATCC stock (VR 1540)
- Treatment groups: Mock, RSV-A2 + Vehicle, RSV-A2 + Ribavirin
- Harvest: Day 5
- Bioburden evaluated
- Consistent Bioassay performed across studies
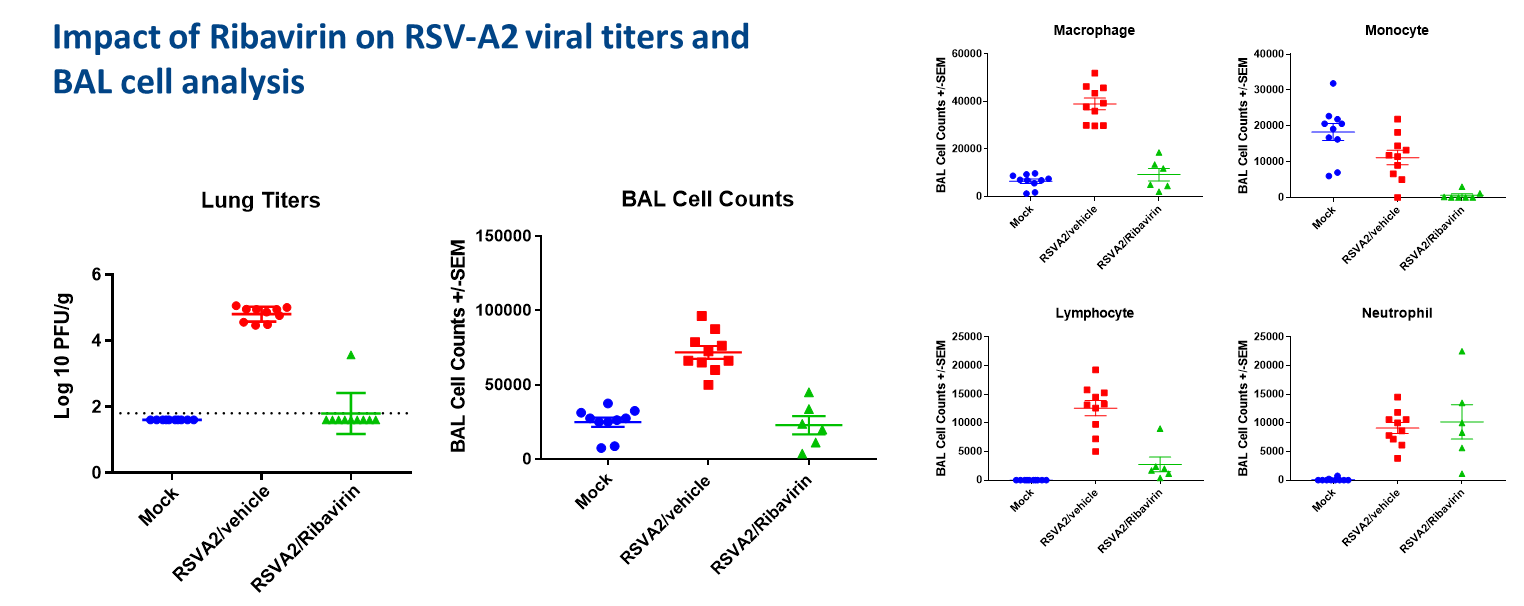
Result
The graphs below demonstrate that Ribavirin treatment reduced the RSV-A2 titers in lung. Ribavirin reduced BAL cell (Macrophages, Monocytes, Lymphocyte and Neutrophil) counts and it also reduced cytokines/ chemokines in BAL fluids.

Methodology
- Female BALB/c: 6-8 weeks of age
- RSV Strain: RSV -A2
- Inoclulum: Intranasal RSV-A2 (1 x 105 pfu)
- Treatment groups: Mock, RSV-A2 + Vehicle
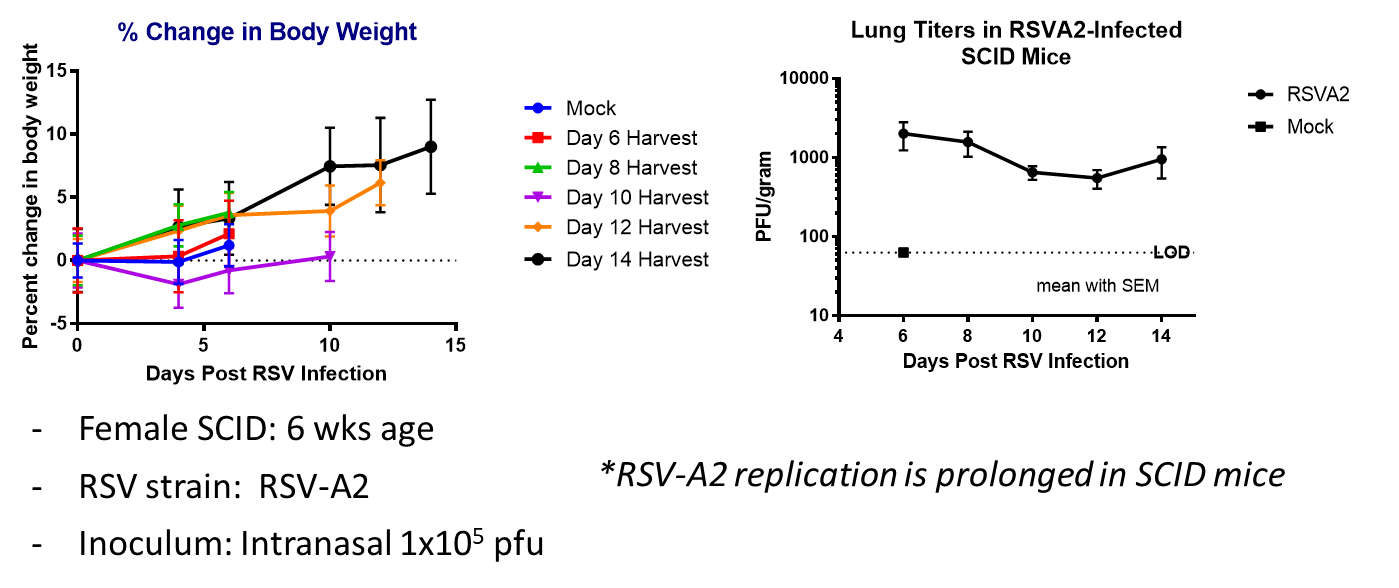
- Harvest: Days post injection (6, 8, 10, 12, 14)
- Change in body weight in RSV-A2 infected mice.
- Lung titers in RSV-A2 infected mice
Result
The graphs show the change in body weight and the RSV-A2 titers in lungs of infected mice over 14 days post infection. RSV-A2 replication is prolonged in SCID mice.
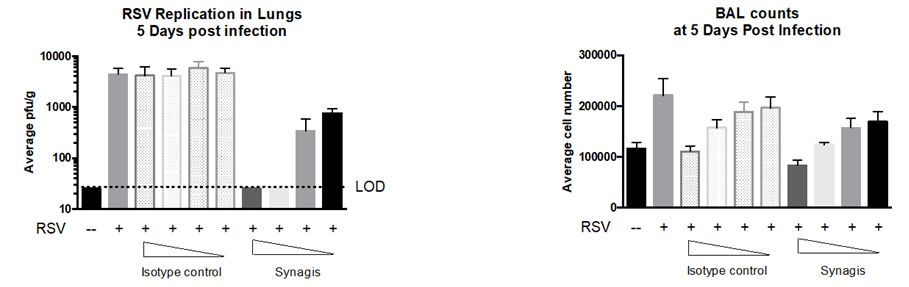
Methodology
- Female Cotton Rats: 6-8 weeks of age
- RSV Strain: RSV Long (ATCC-VR-26)
- Inoclulum: Intranasal
- Treatment on Day 2
- Groups: Mock, Isotype + Vehicle, SYNAGIS® + RSV Long
- Harvest: 4 Days post injection
- Daily Clinical observations
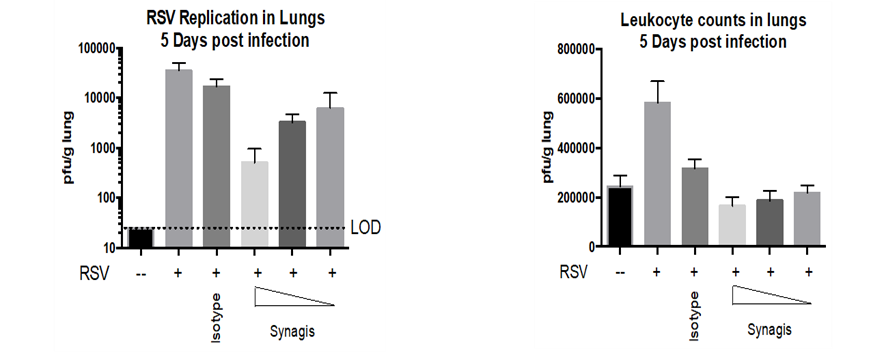
- Lung titers and Leukocyte counts in RSV infected rats.
Result
The graphs below show the RSV replication in lungs and Leukocyte counts 5 days post infection. SYNAGIS® reduced the RSV replication in lungs of the treated cotton rats better than the Isotype-treated group.
Methodology
- Female Cotton Rats: 6-8 weeks of age
- RSV Strain: RSV-A2 (ATCC VR-1540)
- Inoclulum: Intranasal
- Groups: Day 4, Day 5, Day 6
- Harvest (Lungs and Noses): 4, 5, 6 Days post injection
- Daily Clinical observations
- Viral plaque assay on homogenized tissue of rats
Result
Peak RSV replication observed in 4-5 days post challenge in both Lung and Nasal Turbinate.
Mouse Cytomegalovirus: Antiviral Pre-clinical Model
The clinical utility of most anti-CMV drugs is limited by poor oral bioavailability, associated toxicities, and the potential for development of resistance with prolonged use. There is a critical need for novel therapeutic agents to address limitations of FDA approved drugs.
Benefits of the Mouse CMV BALB/c Model
There is a strict species specificity amongst the CMV viruses, making it difficult to evaluate hCMV viral infections in rodent models. Because mCMV and hCMV share similarity at the genetic and nucleotide level and infection of mice with mCMV sets up an infection with many similarities to human disease, the mCMV model has proven to be an excellent model for predicting anti-viral drug efficacy in human CMV disease (Handbook of Animal Models of infection, 1999; chapter 111). It is not as useful for vaccine candidates and biologics.
Aragen offers mCMV preclinical efficacy and safety studies using an appropriate in vivo rodent model to enable development of anti-CMV antiviral drugs.
Experimental mCMV Model for evaluation of anti-CMV vaccines
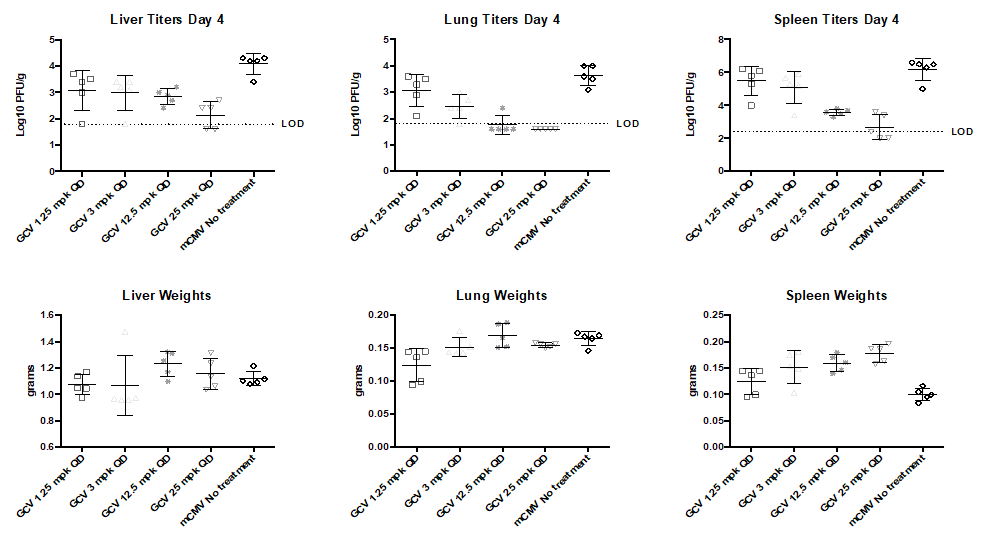
- Day 0: Infect 8-9wk BALB/c mice with desired dose (lethal or non-lethal) of 3X-salivary gland passaged mCMV.
- Monitor weight and survival throughout the study
- Ganciclovir used as positive control/standard of care.
- Day of harvest: Collect salivary glands spleen, liver heart, lungs kidney for titration.
Key Readouts
- Body Weight
- Survival
- Viral titration by plaque assay or qPCR
CMV Case Studies:
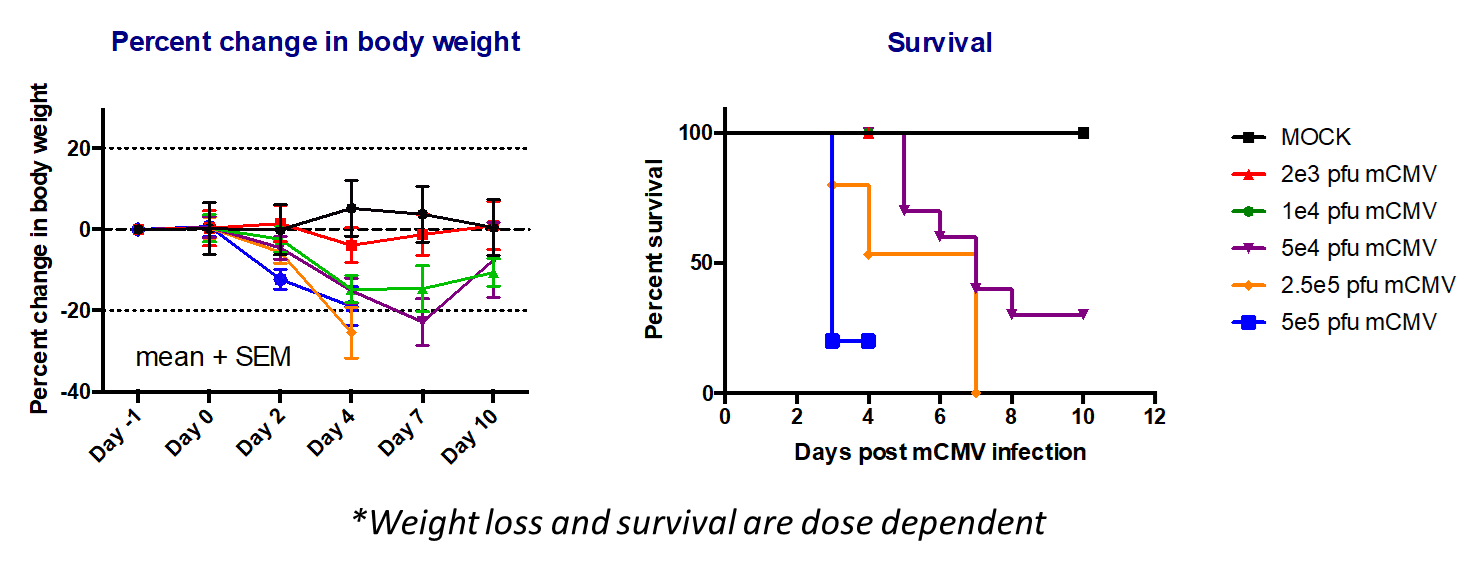
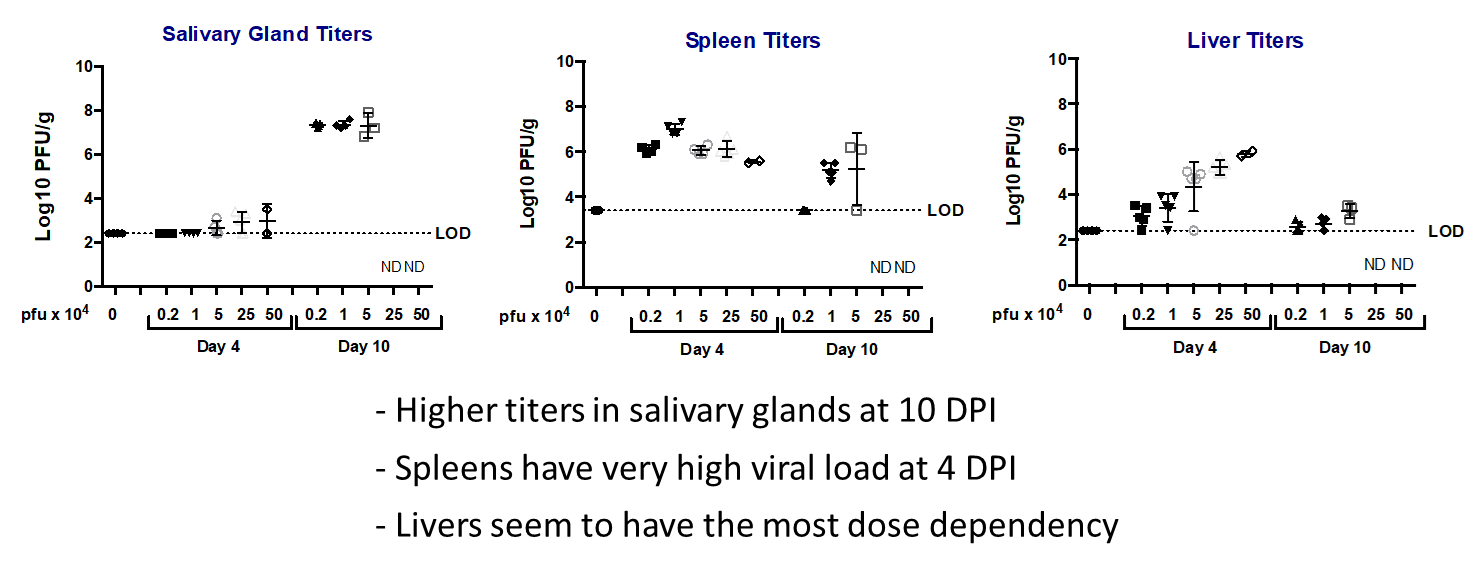
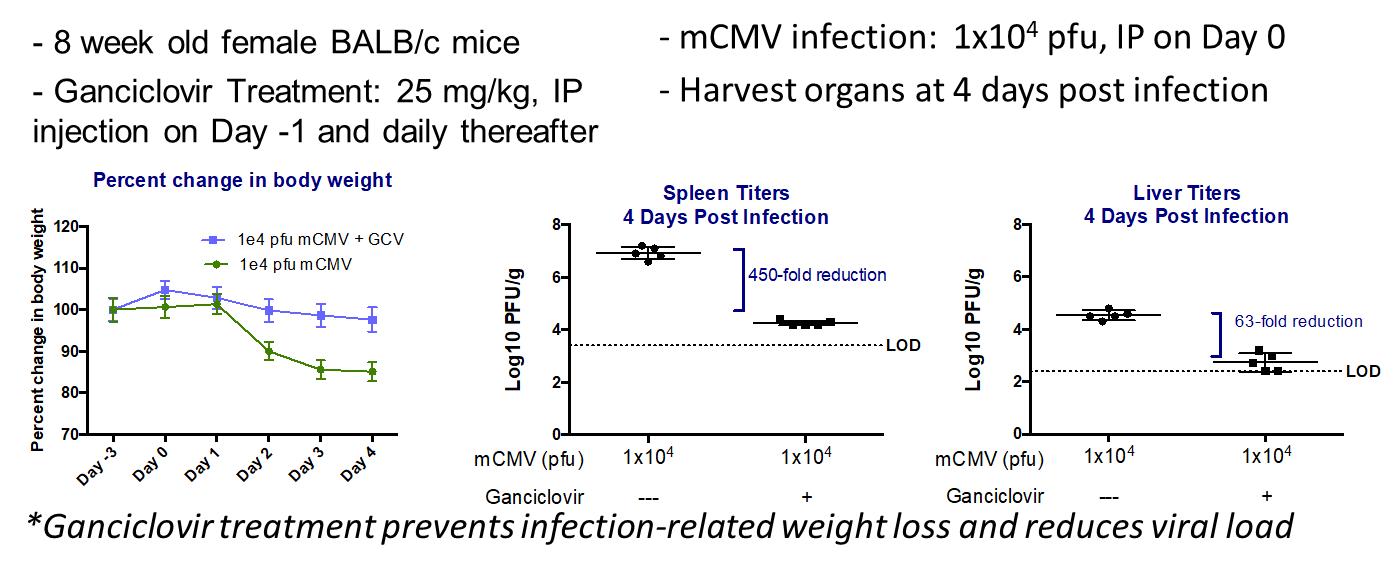
Ganciclovir’s effects on viral titers during mcmv infection
Ganciclovir’s effects on body weights during lethal and non-lethal mcmv infections
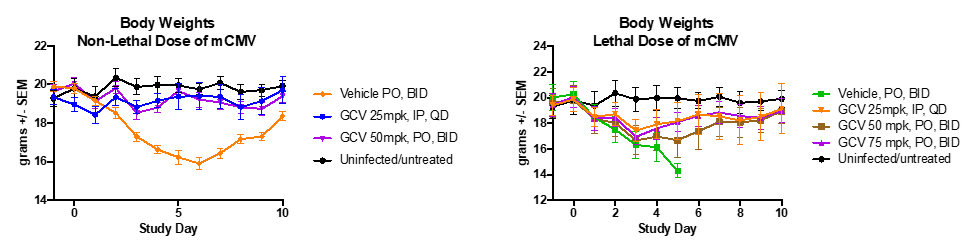
Contact Us to Learn More
Contact our team to learn more about Aragen’s infectious disease models and how we can take your project from immunogen preparation all the way to having an IND-enabling data package.
Select Client References
- In Vivo Efficacy of EDP-323, a Novel L-Protein Inhibitor, for the Treatment of Respiratory Syncytial Virus: Poster and Presentation at 12th International RSV Symposium, 2022, https://www.enanta.com/science/
- Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Frontiers in Immunology. 2021
- Orally administered adenoviral-based vaccine induces respiratory mucosal memory and protection against RSV infection in cotton rats: Vaccine, Volume 36, Issue 29, 2018,
- Direct Inhibition of Cellular Fatty Acid Synthase Impairs Replication of Respiratory Syncytial Virus and Other Respiratory Viruses: PLoS One 2015
- Inhibition of Fatty Acid Synthase in vitro and in vivo Reduces RSV Replication: Poster presentation at ICAAC 2014
- Preclinical development and safety assessment of the first inhaled NANOBODY® ALX-0171: SOT2013
- ALX-0171 toxicity study in RSV-infected cotton rats: safety and therapeutic potential: Poster presentation at Eurotox 2012
- Potent High-Affinity Antibodies for Treatment and Prophylaxis of Respiratory Syncytial Virus Derived from B Cells of Infected Patients: Journal Immunology 2009