Protein Analytics and Characterization Highlights
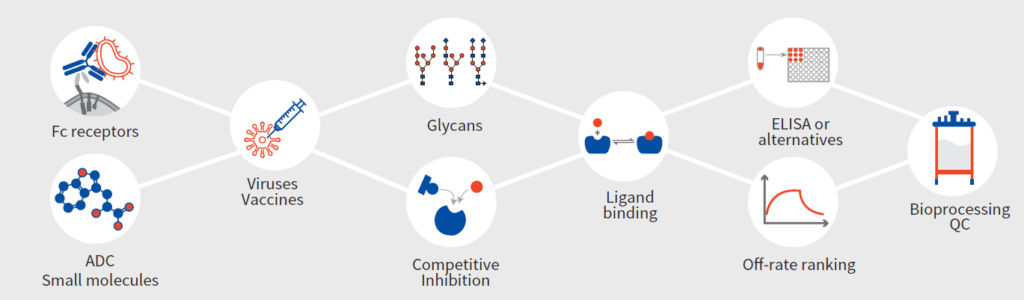
- Assess and rigorously test the quality of purified target molecules.
- CE-SDS, cIEF, SEC-HPLC/MALS, SDS-PAGE, LC-MS, DLS/SLS, LAL, HIC, HILIC
- Establish assays and screens for interactors and/or inhibitors.
- SPR BLI, Multimode plate readers
- Validate hits and characterize various interactions of drugs.
- SPR, BLI, Multimode plate readers
- Characterize final drug products for FDA approval or patent preparation.
- cIEF, CE-SDS, SEC-HPLC/MALS, DLS/SLS, LAL, HILIC, LC-MS, SPR, BLI, Multimode plate readers
- Intact Mass non-reduced, reduced, +/-PNGaseF (LC-MS/QToF)
- PepMap, PepMap UV (LC-MS/QToF)
- N-Glycan profiling by RP-MS or HILIC-MS (LC-MS/QToF)
- Glycation (LC-MS/QToF)
- Sialic Acid Content (UPLC)
- N -Glycan profiling (HILIC – FLD/UPLC and CE-SDS)
- Size variants (including aggregates) (SDS-PAGE)
- Hydrophobic variants (RP-HPLC/UPLC or HIC)
- Free – thiol (SEC-FLD)
- Charge variants (cIEF, CZE or Slab Gel IEF)
- Western blot
- Binding kinetics (kon, koff, Kd)
- FcR and C1q screening
- Functional blocking
- Epitope binning
- Accurate mass for proteins, protein-drug conjugates
- Peptide and glycan mapping
- Tm, Tagg, Size and PDI
- Isothermal stability
- Thermal recovery
- Sizing and polydispersity
- Sizing and thermal ramp
- B22 and kD
- Viscosity
- Protein concentration by Protein-ARP
- A280
- Protein/antigen titer (BLI)
- Residual protein A/G/L (ELISA or Octet)
- Host cell proteins (Cygnus) (ELISA)
- Endotoxin testing (LAL/ Charles River EndoSafe)
- Bioburden, Sterility (Culturing)
Octet

Biacore

Uncle
Leveraging the Latest Technology Platforms
Aragen’s team leverages state-of-the-art instrumentation to achieve the desired analytical results with the throughput needed to meet your development timeline. Our lab relies on protein purification chromatography systems (Cytiva ÄKTA) and tangential flow filtration systems (KrosFlo TFF). Our fully-equipped analytical lab includes LC-MS/QToF (Agilent 1290 UPLC + 6530B QToF), UPLC (Agilent), SPR (Cytiva Biacore), BLI (Sartorius Octet) , MALS, DLS/SLS (Unchained Labs Uncle), CE (Sciex) and Multimode microplate readers (Molecular Devices SpectraMax).
Developability Assessments to Enable Effective Clinical Strategies
Developability is a term often used to describe the evaluation process applied to lead molecules for their potential to be developed into drug products. Developability studies encompass a rigorous set of biochemical and biophysical tests to determine the candidate’s critical quality attributes (CQA) that are necessary for functionality and stability. This process allows the investigator to identify candidates to move forward and to exclude or redesign those with unfavorable profiles, thus avoiding potential pitfalls during the lifecycle of a biotherapeutic. At Aragen, we provide a suite of services available for the customer to identify their CQA and test how those attributes are susceptible to a particular stress.
Developability Studies
Our developability assessment expertise helps identify the physical and chemical properties of drug candidates, such as aggregation, post-translational modifications, stability, and solubility, that can have an adverse impact on the safety, efficacy, manufacturability and ultimately the success of clinical candidates. In the Case Study below, Aragen scientists examined two molecules that were lead candidates in a study that underwent a number of stressors prior to proceeding in the developability pipeline, selecting the candidate with more favorable properties.
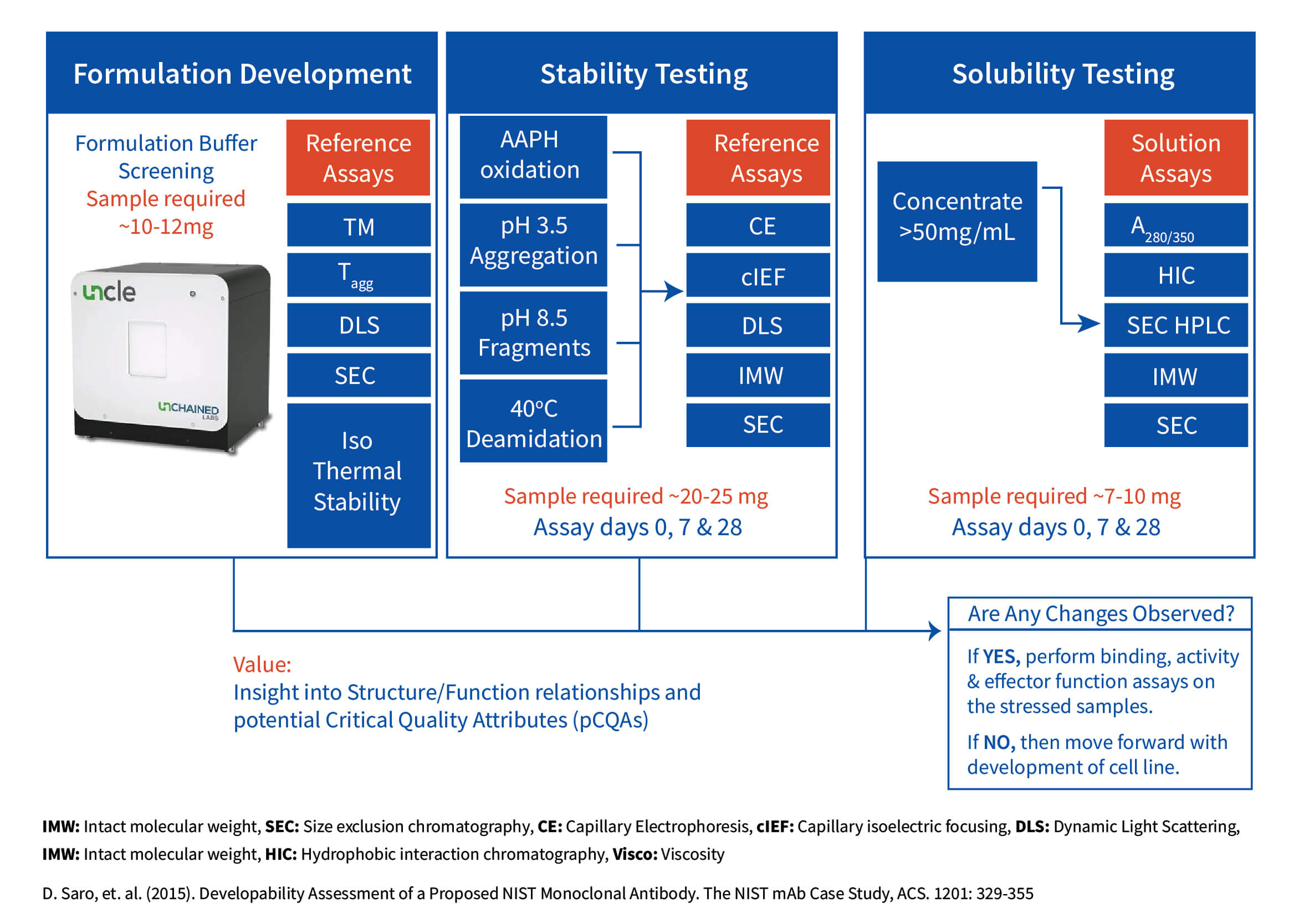
The stability of any lead candidate(s) is a requisite for its potential biotherapeutic application. An approach to assess the integrity of the molecule is to perform a heat-stress study that can accelerate and highlight any instability issues that may be present. For the example below, two molecules were lead candidates in a study that underwent a number of stressors prior to proceeding in the developability pipeline. The heat-stress data displayed stark differences in their stability profiles.
Lead candidates were subjected to a 28-day heat stress at 40°C followed by charge variant (cIEF) and size variants (CE-SDS) analytics. The heat stress study demonstrated that both molecules undergo changes in their charge variants (Figure 1). Comparing Day 0 (black traces) to Day 28 (red traces) the main peak species for both molecules decreased and shifted to more acidic species (increase in the distribution of acidic peaks), suggesting that oxidation and deamidation are likely occurring. This is observed in both molecules, but to a greater extent on lead candidate #1 suggesting that this molecule is more susceptible to heat stress than candidate #2.
Using size variant (CE-SDS) analysis in both reducing (Figure 2) and non-reducing (Figure 3) formats, we observed that the main peaks for candidate #1 diminish more extensively than candidate #2. Specifically, candidate #1 main peak in the reduced format shows that there are additional species or fragments present on Day 28 that are not present on Day 0 (Figure 2, left). This is further supported by non-reducing CE-SDS (Figure 3, left panel) where the intact main peak at Day 0 has deteriorated by Day 28 at 40°C suggesting fragmentation of both light and heavy chains. In contrast, lead candidate #2 demonstrated minimal fragmentation retaining the overall size variant profile at Day 28 as was present at Day 0 (Figures 2 and 3, right panels). In conclusion, the advancement of lead candidate #2 suggests having a higher likelihood of success through the development process, where oxidation or deamidation may be mitigated through the addition of excipients.
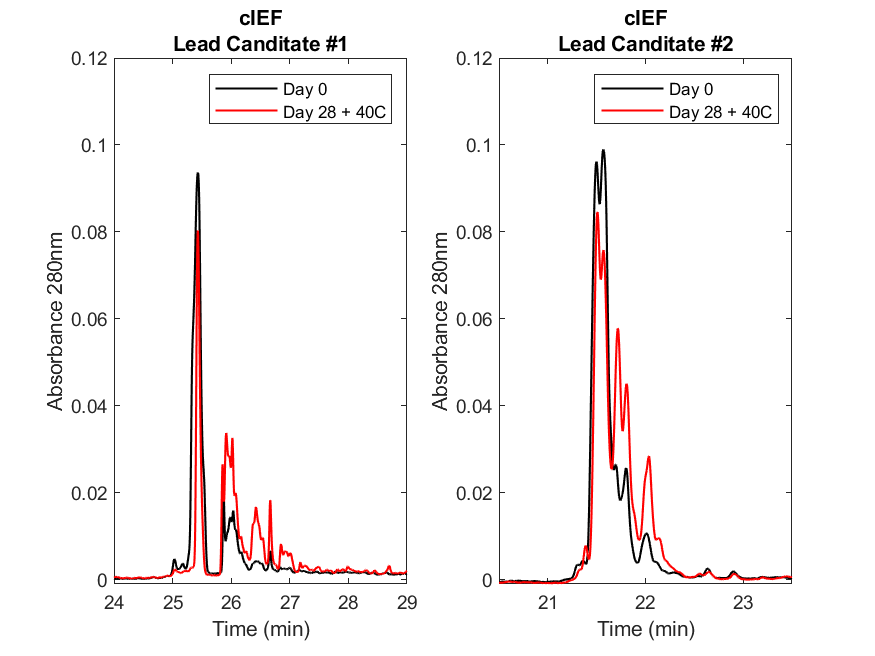
Figure 1. Charge variant (cIEF) analysis of two lead molecules demonstrates the effects of a heat stress study.
The cIEF electropherograms for lead candidate #1 (left) and #2 (right) at Day 0 (black) and after 28 days at 40°C (red). On Day 0, the lead candidate #1 abundance of the main peak (~25.3-25.7 min) with a pI of 8.7 is ~75% then drops to ~30% by Day 28. For lead candidate #2, the abundance of the main peak (~21.4-21.6 min) with a pI of 9.5 is ~79% then drops to ~63% by Day 28. Results were collected on a Sciex PA800 Plus system, analyzed using the 32Karat software, and figures generated using MATLAB (R2019b).
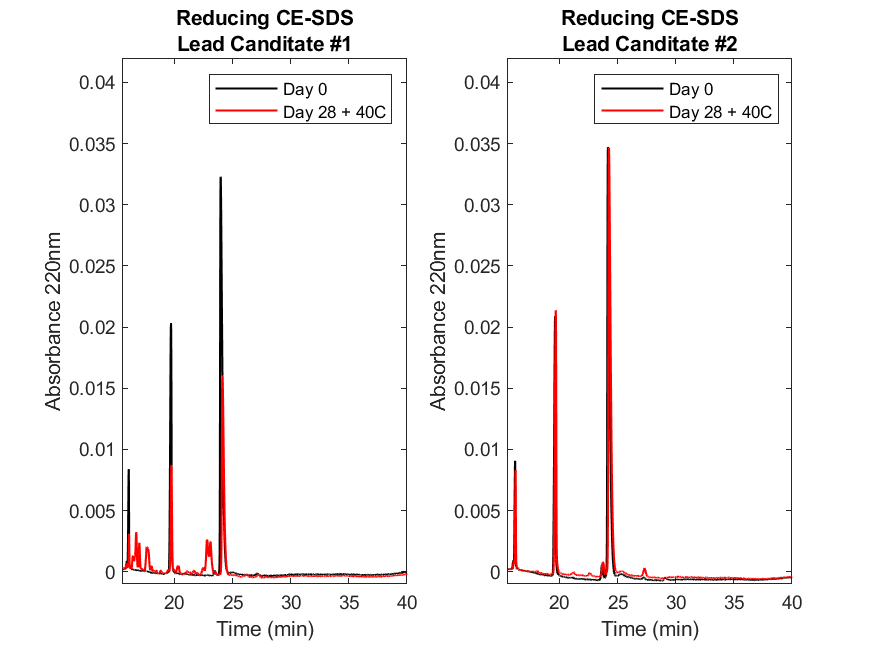
Figure 2. Reducing size variant (CE-SDS) analysis of two lead molecules demonstrates the effects of a heat stress study.
The reducing CE-SDS electropherograms for lead candidate #1 (left) and #2 (right) at Day 0 (black) and after 28 days at 40°C (red). On Day 0, the lead candidate #1 abundance of the main peaks comprising of light (~19 min) and heavy (~24 min) chains was ~97%, but drops to ~53% by Day 28. Notably, there are fragments in between the light and heavy, indicating fragmentation of the heavy chain. For lead candidate #2, the abundance of the light (~19 min) and heavy (~24 min) chains remain high starting ~99% then drops to ~97% by Day 28 suggesting the molecules remain intact throughout the stress study. Results were collected, analyzed, and figures generated as noted in the legend of Figure 1.
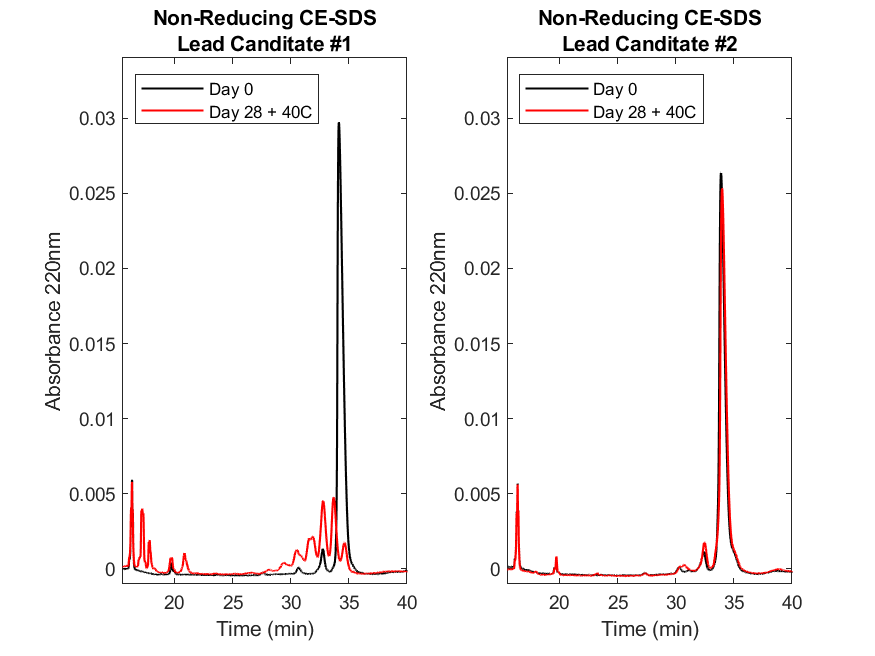
Figure 3. Non-reducing size variant (CE-SDS) analysis of two lead molecules demonstrates the effects of a heat stress study.
The non-reducing CE-SDS electropherograms for lead candidate #1 (left) and #2 (right) at Day 0 (black) and after 28 days at 40°C (red). On Day 0, the lead candidate #1 abundance of the main peaks comprising of the intact molecule (~34 min) starts at ~96% but drops to ~7% by Day 28. Unfortunately for the lead candidate #1, the fragmentation (~16-33 min) of the main species is observed by Day 28. For lead candidate #2, the abundance of the intact molecule (~34 min) remains high starting ~92% then drops to ~88% by Day 28 suggesting the molecules remain intact throughout the stress study. Results were collected, analyzed, and figures generated as noted in the legend of Figure 1.
Contact Us to Learn More
Whether you are focused on lead discovery, production of targets for high throughput screening (HTS), or characterization of unique gene products or antibodies, Aragen has the expression, purification and analytical capabilities to meet your needs. Contact our team in the Morgan Hill, California facility to learn more.
