We specialize in providing end-to-end analytical solutions for a wide range of biotherapeutic modalities, including:
- Monoclonal, bispecific, and multi-specific antibodies
- Fusion proteins
- Enzymes
- Recombinant proteins
- Antibody drug conjugates (ADCs)
Our Capabilities
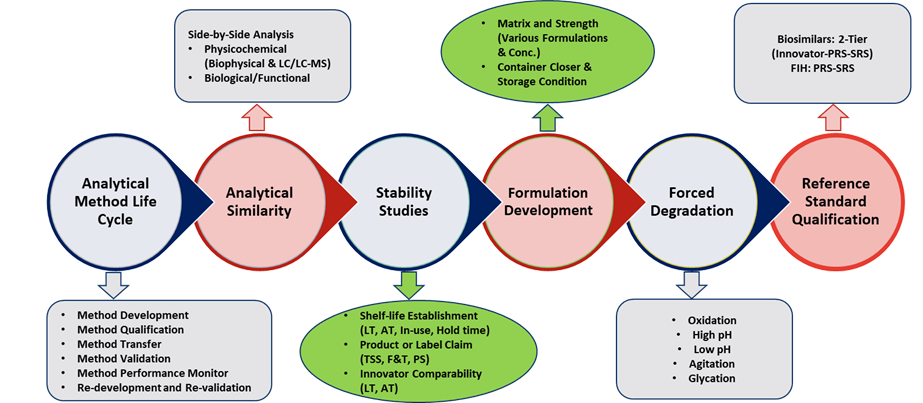
Our Analytical Services
- Platform Methods
- Routine Analytical Testing
- Product Characterization
- Bioassay Method Development
- Stability Studies & Forced Degradation
- Reference Standard Qualification (IRS/PRS/SRS)
- Method Life Cycle Management
Each service is tailored to meet your product’s unique needs and is designed to ensure quality, safety, and compliance throughout the biologic lifecycle.
Why Aragen?
A Partner You Can Trust
- Breadth of Expertise: Our analytical services cater to both standard and complex biologics, including multi-functional proteins, ensuring precise and reliable results for a wide range of biologic products.
- Dedicated Facilities: Our facilities are designed to provide precision, speed, and compliance, ensuring that your biologic product is tested to the highest industry standards.
- Advanced Analytical Tools: We utilize advanced tools, including LC-MS, CAD, DSF, SPR, and DLS to ensure the highest precision, quality, and reproducibility in every phase of testing.
- Qualified and Validated Equipment: All equipment used in our analytical development is qualified and validated in accordance with industry guidelines (GAMP, CSV, etc.), ensuring regulatory compliance and reliable results.
- End-to-end Support: From method development and early-stage research to GMP validation and post-market stability studies, we provide integrated, seamless support throughout the entire product lifecycle.
- Regulatory Expertise: Our services are aligned with global guidelines such as ICH Q1B, ICH Q2R2, ICH Q5C, ICHQ9R1, ICH Q14 & FDA standards, ensuring that your biologic meets all necessary regulatory requirements for timely approvals.
With Aragen, you gain a trusted partner who provides expertise, regulatory readiness, and end-to-end support to accelerate your biologic’s development, reduce risks, and ensure a smooth path to commercialization. Contact us today to learn how Aragen’s Analytics Services for Biologics can support the success of your biologics product’s journey from development to commercialization.

