Built Around Your Biologics Journey
Whether you are developing a novel therapeutic or advancing a biosimilar, our solutions are designed with your goals in mind. From host cell line selection to IND-ready packages, we support every stage of your development.
When you choose Aragen, you are partnering with a leader that works as an extension of yours—focused on solving your biggest technical challenges while unlocking the full potential of your product.

What Sets Our CLD Services Apart?
We do not believe in one-size-fits-all. Our approach is flexible, efficient, and fully customized to your biopharmaceuticals, your timelines, and your success.
- Broad Host Cell Line and Vector Capabilities: We are proficient in working with multiple host cell lines including CHO DG44, CHO GS, SP2/0, and NS0, and we support various expression systems such as DHFR, GS, and antibiotic resistance vectors.
- Deep Expertise Across Biologics: We have successfully expressed a wide range of proteins including human, mouse, canine, and feline IgGs, fusion proteins, enzymes, cytokines, hormones, mini-bodies, and bispecific antibodies.
- RapTr2022: Fast-Track Platform for Complex Projects: Our RapTr2022 platform is built for speed and performance. Leveraging our proven CHO-DG44 and CHO-GS systems, it is ideal for clients needing to express difficult proteins or meet accelerated timelines.
- Three Decades of Scientific Depth: We bring more than 30 years of expertise in engineered cell lines and biologics development—ensuring your product is built on a foundation of science and strategy.
Your Fast Track to IND: Seamless, Stable Cell Line Development
Bringing your biologic to market starts with a stable, high-yielding cell line. Our streamlined process is designed to move you efficiently from DNA to Research Cell Bank (RCB)—helping you file your IND with confidence.
With over 200 successful CLD projects and 100+ advancing to clinical phases, we have proven our ability to deliver high-quality results, fast.

Platform Overview: Scalable, Regulatory-Ready Solutions
Aragen Bioscience offers a full spectrum of cell line development services that ensure robust, scalable, and regulatory-compliant production.
CHO-DG44 Platform
Trusted for stability and high productivity—an ideal choice for biosimilar development.
- Royalty-free and low-risk
- >4g/L protein yield in ~18 weeks
- IND-/BLA-ready data packages
- Expertise in biosimilar development
CHO-GS Platform
Robust and versatile, built around a proprietary GS-expression vector.
- Royalty-free, stable expression platform
- Mini pools up to 4.2g/L | Bulk pools up to 700mg/L
- Single cell clones (SCC) up to 8g/L in fed-batch flasks
- 60+ days of stability in growth and productivity
- Optimized from transfection to SCC in just 16 weeks
RapTr2022: The Express Lane to IND
If time is your top priority, RapTr2022 delivers.
- Royalty-free, cost-efficient, and fast
- Up to 8g/L protein expression
- DNA transfection to Research Cell Bank (RCB) in just 18 weeks
- Advanced imaging ensures clonality and consistency
- Built for fast-track biologics and accelerated IND filings
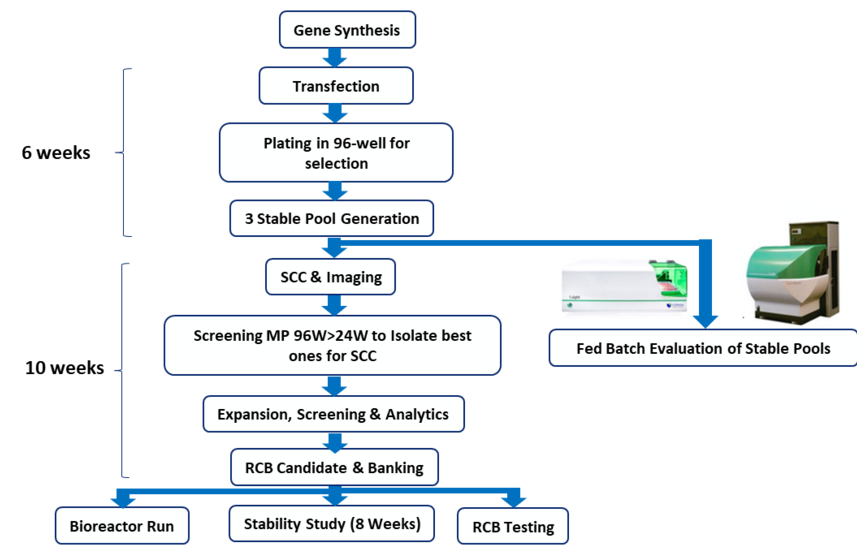
CLD Workflow
Parallel Cell Line Development: Reduce Risk, Maximize Return
Choosing the right expression platform can be a make-or-break decision. We mitigate risk by offering parallel development—testing multiple platforms in tandem so you can identify the best-performing line early. Once a lead is confirmed, alternate tracks are paused, saving time and resources without delaying your IND.
Specialized Biosimilar Support
As the biosimilar market continues to grow, we help you capitalize on it. Our team is equipped to manage the scientific and regulatory complexity of biosimilar development with precision.
- Structural Equivalence: Develop biosimilars that mirror innovator drugs in safety, structure, and efficacy
- Process Optimization: Tailored solutions for scale-up and cost-effective production
- Therapeutic Area Expertise: Inflammatory diseases, oncology, rare genetic disorders, and more
The Aragen Advantages
We’re more than a vendor—we’re a long-term partner invested in your product’s success.
- 200+ successful CLD programs
- 100+ IND-enabling projects
- 5-month CLD timelines for faster market entry
- Diverse platform offerings: CHO DG44, CHO GS, RapTr2022
- Proprietary vectors and analytical rigor
- Bioreactor evaluation and long-term stability studies
- Full CMC support from clone selection to tech transfer
- Expertise in therapeutic, diagnostic, and veterinary biologics
- Parallel development reduces risk and budget pressure
- Generated clonal cell lines
- Evaluated single cell clones in fed-batch shake flask
- Scalable in bioreactor







|
IgG Epidermal Growth Factor Receptor Blocker (HER-2 Type) or Rec-MC Antibody |
|||
|
HPCL-SEC Purity |
|||
|
Sample ID |
Main Peak |
HMWS |
LMWS |
|
1 |
98.80 |
1.20 |
0.00 |
|
2 |
97.50 |
2.11 |
0.39 |
|
3 |
97.81 |
1.81 |
0.38 |
|
4 |
97.73 |
1.91 |
0.37 |
|
5 |
97.97 |
1.56 |
0.47 |
|
6 |
98.85 |
1.15 |
0.00 |
|
7 |
96.88 |
2.66 |
0.47 |
|
8 |
97.66 |
1.96 |
0.39 |
|
9 |
98.84 |
0.73 |
0.43 |
|
10 |
97.73 |
1.84 |
0.44 |
|
IgGA Control |
|||
|
IgGA Start |
99.10% |
0.90% |
0.00% |
|
IgGA End |
99.10% |
0.90% |
0.00% |
- Stable Bulk Pool titers achieved~700mg/L
- Single cell clone (SCC) from Bulk Pool reached up to 2g/L with clones showing stability up to 60 days
- Mini pool (MP) generated, and MP reached up to 4.2g/L
- SCC were generated from the top 2 mini-pools and fed-batch shake flask reached up to 6 g/L
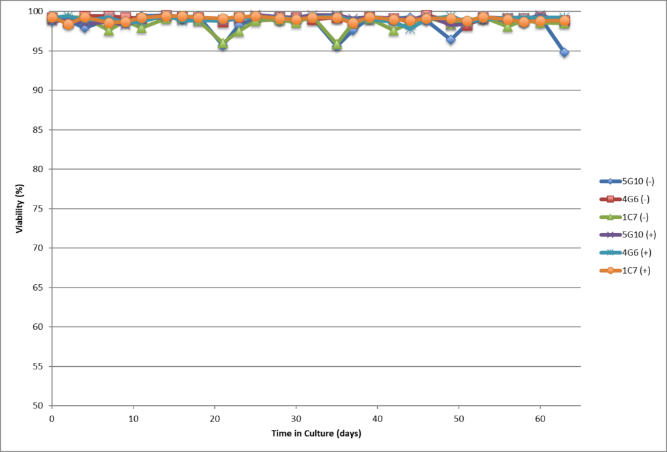
- Single cell clones show stability over 60 days in respect to cell growth, maintaining high viability and specific productivity
- Using Aragen’s optimized process from transfection to Single cell clone evaluation completed in 18wks


|
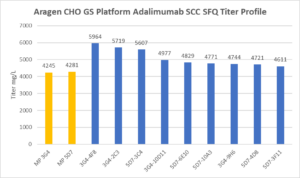
Summary Table for Aragen Bioscience CHO GS Anti-inflammatory Tumor Necrosis Factor Inhibiting Agent MP SFQ |
|||
|
MP ID |
% Viability at Harvest |
PVCD E6 c/mL |
Harvest Titer (mg/L) |
|
5D7 |
91 |
22 |
4281 |
|
3G4 |
88 |
16 |
4245 |
|
1A10 |
83 |
14 |
2961 |
|
3E5 |
93 |
17 |
2728 |
|
4B6 |
84 |
13 |
1123 |
|
3G7 |
90 |
12 |
1065 |
|
4C2 |
69 |
9 |
687 |
|
4E2 |
86 |
12 |
539 |
Single cell clone generation and evaluation
- The Top two MPs were used to generate SCC
- SCC in fed-batch shake flask evaluation reached up to 6g/L
|
Summary Table for Aragen Bioscience CHO GS Anti-inflammatory Tumor Necrosis Factor Inhibiting Agent MP SFQ |
|||
|
MP ID |
% Viability at Harvest |
PVCD E6 c/mL |
Harvest Titer (mg/L) |
|
5D7 |
91 |
22 |
4281 |
|
3G4 |
88 |
16 |
4245 |
|
1A10 |
83 |
14 |
2961 |
|
3E5 |
93 |
17 |
2728 |
|
4B6 |
84 |
13 |
1123 |
|
3G7 |
90 |
12 |
1065 |
|
4C2 |
69 |
9 |
687 |
|
4E2 |
86 |
12 |
539 |
Stability study of Single Cell Clones (SCC) from Bulk Pool (BP)
- Completed stability study on top 3 SCC from BP
- Completed 60 days stability both with and without selection
- High viability, good growth rate, shorter dT
- All three SCC showed stable specific productivity up to 60 days