Stable Cell Line Development at Aragen – Accelerating speed to IND (Investigational New Drug)
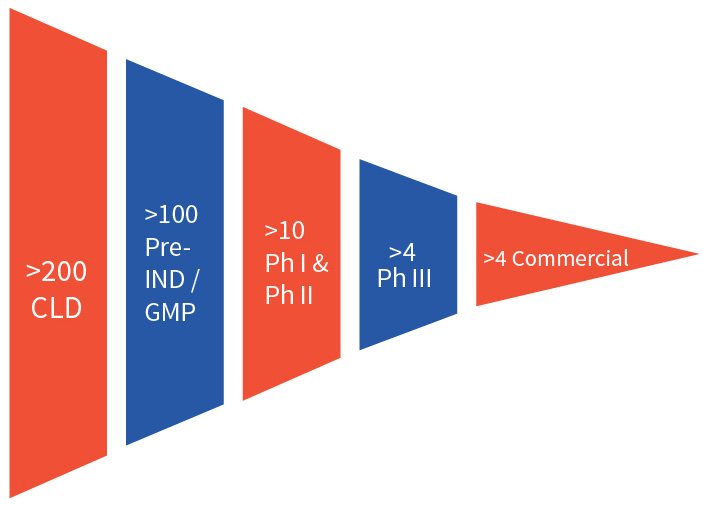
Efficient cell line development is critical in bringing biologics to market and requires a team of experienced scientists, a portfolio of well validated cell line platforms, and ultramodern facilities. Aragen delivers this combination and has completed more than 200 cell line development projects, with over 100 of those cell lines in the clinic following an investigational new drug (IND) application. Aragen has successfully developed over four cell lines that are used in marketed products.
Support for a broad range of host cell lines and expression vectors
Aragen’s researchers are specialists in handling a wide range of host cell lines (CHO DG44, CHO GS, SP2/0, and NS0) and expression vectors (DHFR, Glutamine Synthetase (GS), and antibiotics).
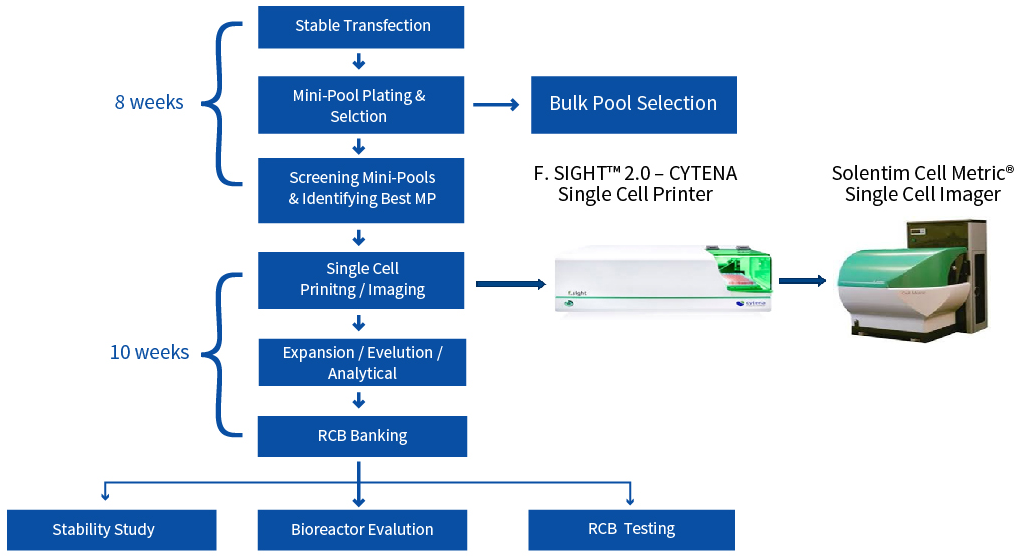
CLD Process Flow
RapTr2022 with Higher titer and Shorter Timeline – A royalty-free CHO-DG44 CLD
Key Highlights
Our Biologics team has produced a range of biologics, including human, mice, canine, and feline IgGs, fusion proteins, enzymes, hormones, cytokines, mini bodies, and bispecific antibodies with this platform. RapTr2022 is best suited to produce difficult-to-express proteins.
A strong record of accomplishment in successfully generating cell lines to deliver more than 200 CLD projects. The majority of these CLD projects resulted in IND applications leading to clinical development.
Our resources and capabilities fit your unique requirements at each step of the CLD process. Our RapTr2022 platform should expedite your biotherapeutic product journey to clinic because it minimizes risk and maximizes efficiency.
CHO DG44
Our internal CHO DG44 platform is essentially free-to-own (royalty-free) and is a high- productivity CHO cell line choice that can deliver >4g/L in 5 months for a range of biologics. The DG44 platform has an extensive regulatory record of accomplishment and uses commercially available media and feeds.
Highlights
- Royalty-free CHO DG44 platform
- Production of 4-5 g/L protein in ~18 wks.
- IND- / BLA-ready packages
- 50% antibodies.
- 50% non-antibody proteins
- Expertise in generating Biosimilar Drugs
CHO GS Platform with Higher titer and shorter Timeline for Antibody Production
The GS-CHO expression system, a protein-free-adapted CHO-K1-derived cell line employs the glutamine synthetase (GS) gene expression system and is extensively used for biotherapeutic production.
Key Aspects of our CLD platforms :
- A royalty-free CHO GS Cellline Development (CLD) platform
- CHO GS- stable cell line expression platform to produce proteins and antibodies.
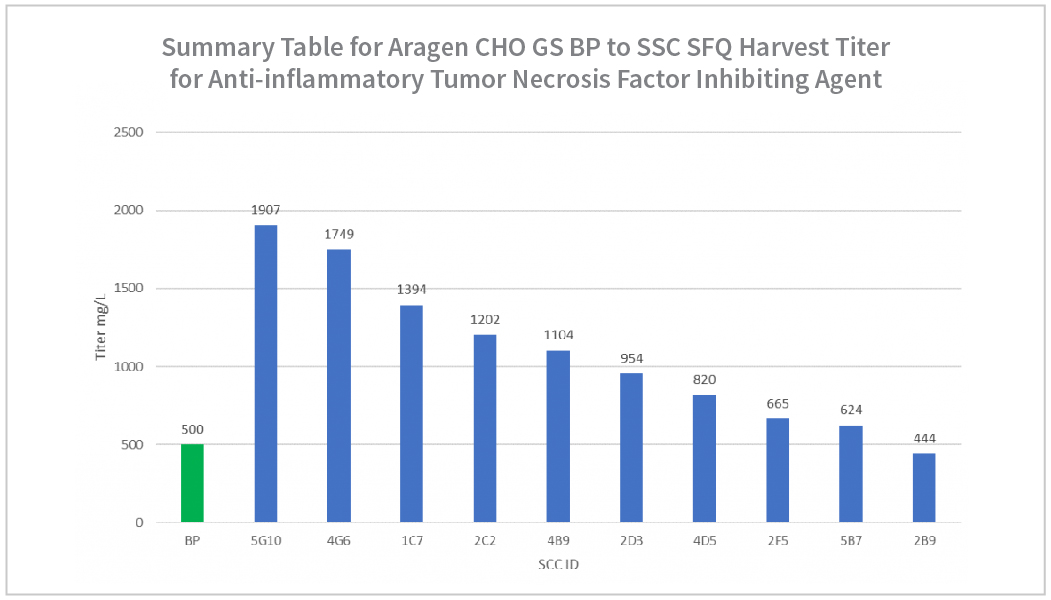
- Stable bulk pool (BP) evaluation reached ~700mg/L.
- Mini pool (MP) generated, and MP reached up to 4.2g/L.
- SCC (Single Cell Clones) were generated from the top 2 mini-pools and fed-batch shake flask reached up to 6 g/L.
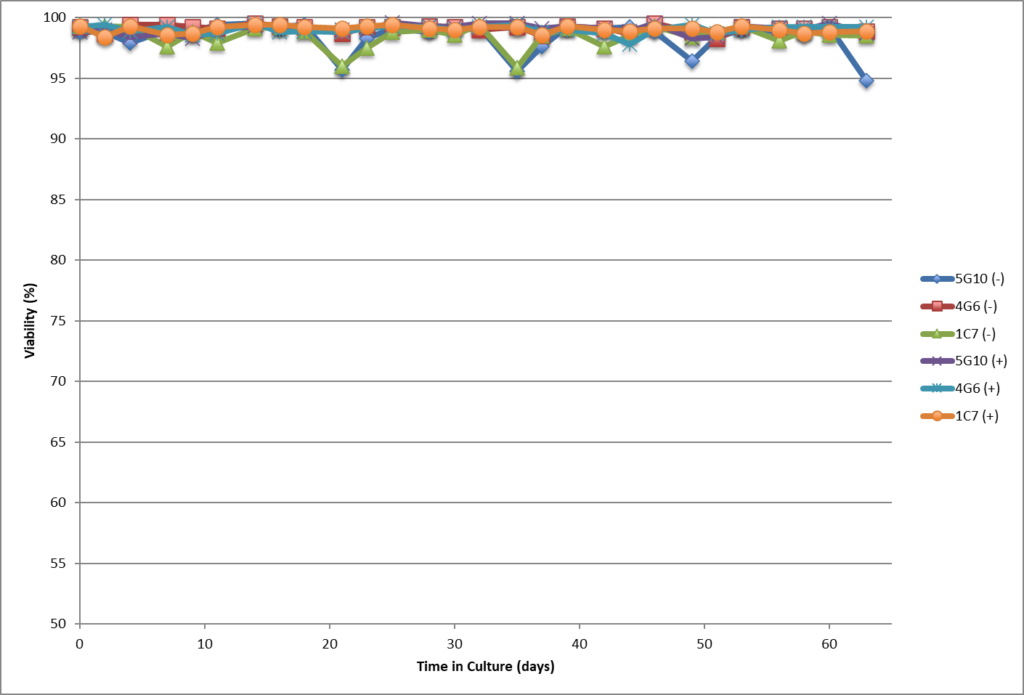
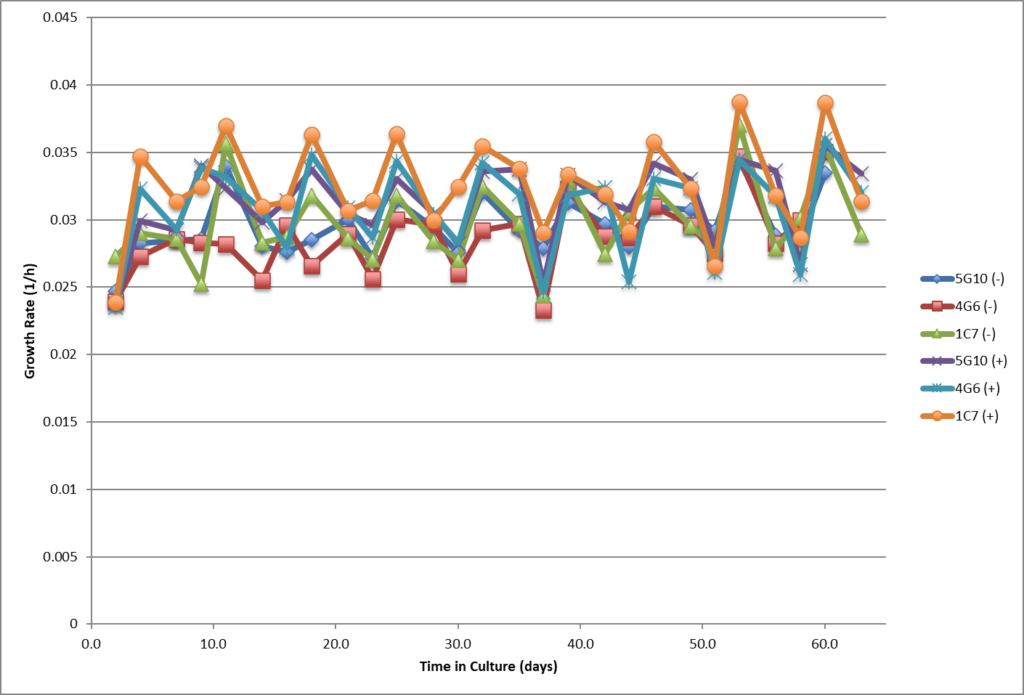
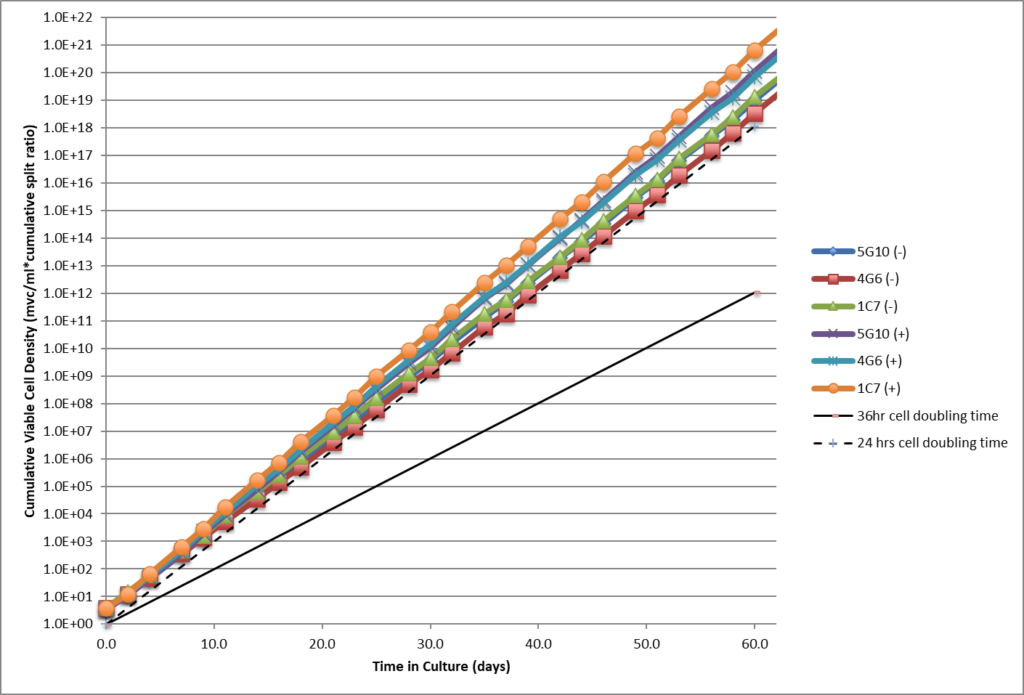
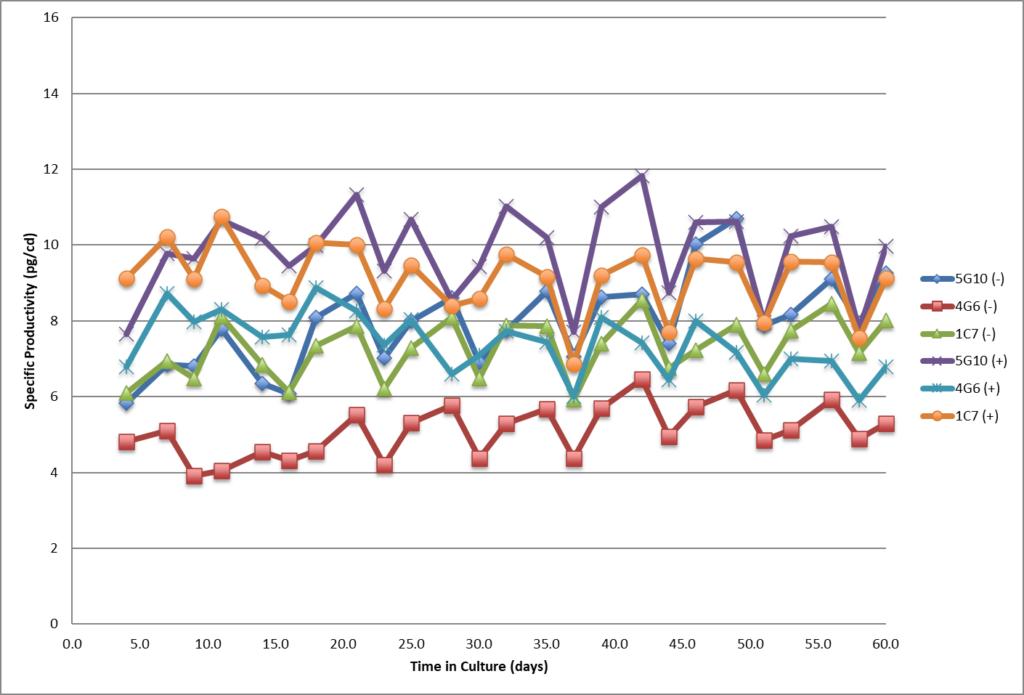
- Single cell clones show stability over 60 days in respect to cell growth, maintaining high viability and specific productivity.
- Using Aragen’s optimized process from transfection to Single cell clone evaluation completed in 18wks.
Sigma’s CHOZN
Sigma’s CHOZN platform is based on deletion of the CHO glutamine synthetase (GS) gene with Talon gene editing technology. The resulting GS-/- CHO host and expression vector with GS choice is a great combination for companies with an interest in an established GS selection system. Regulators are familiar with its efficacy and operation, and its expression vector produces titers equivalent to the DG44 platform.
Mitigating risk with parallel cell line development
Aragen’s cell line development solutions provide cost-effective approaches to test different platforms simultaneously without delaying IND filing timeline. We improved breaks in the CLD process so that parallel work can be stopped as soon as data is available. This helps in the selection of the most effective platform.
Cell Line Services for Biosimilar Development
- Due to patent expiry of branded therapeutics a significant opportunity has emerged for the development of biosimilar drugs.
- We have partnered with clients to generate biosimilars with demonstrable structural equivalence to that of innovator drugs (like post-translational modifications-glycosylation)
- Optimized the manufacturing scale-up with tight control and tailored analytics to ensure that the biosimilar fits innovator drug specifications.
- Working with small to large clients developing biosimilar products for inflammatory disease, genetic disorders, and cancer.
- Generated clonal cell lines
- Evaluated single cell clones in fed-batch shake flask
- Scalable in bioreactor
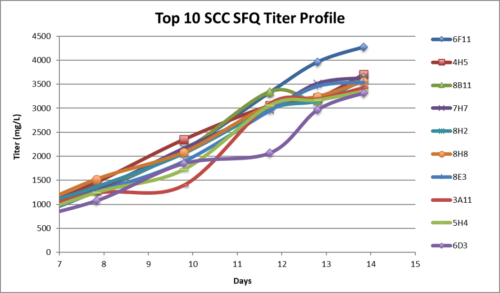
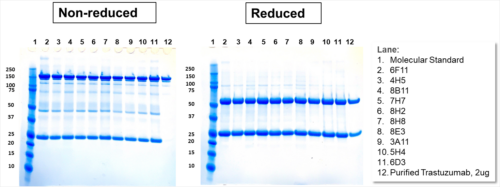
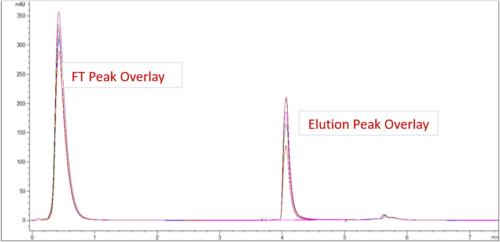
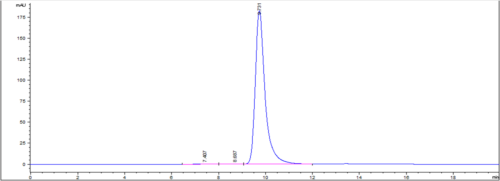
| IgG Epidermal Growth Factor Receptor Blocker (HER-2 Type) or Rec-MC Antibody | |||
|---|---|---|---|
| HPCL-SEC Purity | |||
| Sample ID | Main Peak | HMWS | LMWS |
| 1 | 98.80 | 1.20 | 0.00 |
| 2 | 97.50 | 2.11 | 0.39 |
| 3 | 97.81 | 1.81 | 0.38 |
| 4 | 97.73 | 1.91 | 0.37 |
| 5 | 97.97 | 1.56 | 0.47 |
| 6 | 98.85 | 1.15 | 0.00 |
| 7 | 96.88 | 2.66 | 0.47 |
| 8 | 97.66 | 1.96 | 0.39 |
| 9 | 98.84 | 0.73 | 0.43 |
| 10 | 97.73 | 1.84 | 0.44 |
| IgGA Control | |||
| IgGA Start | 99.10% | 0.90% | 0.00% |
| IgGA End | 99.10% | 0.90% | 0.00% |
- Stable Bulk Pool titers achieved~700mg/L
- Single cell clone (SCC) from Bulk Pool reached up to 2g/L with clones showing stability up to 60 days
- Mini pool (MP) generated, and MP reached up to 4.2g/L
- SCC were generated from the top 2 mini-pools and fed-batch shake flask reached up to 6 g/L
- Single cell clones show stability over 60 days in respect to cell growth, maintaining high viability and specific productivity
- Using Aragen’s optimized process from transfection to Single cell clone evaluation completed in 18wks
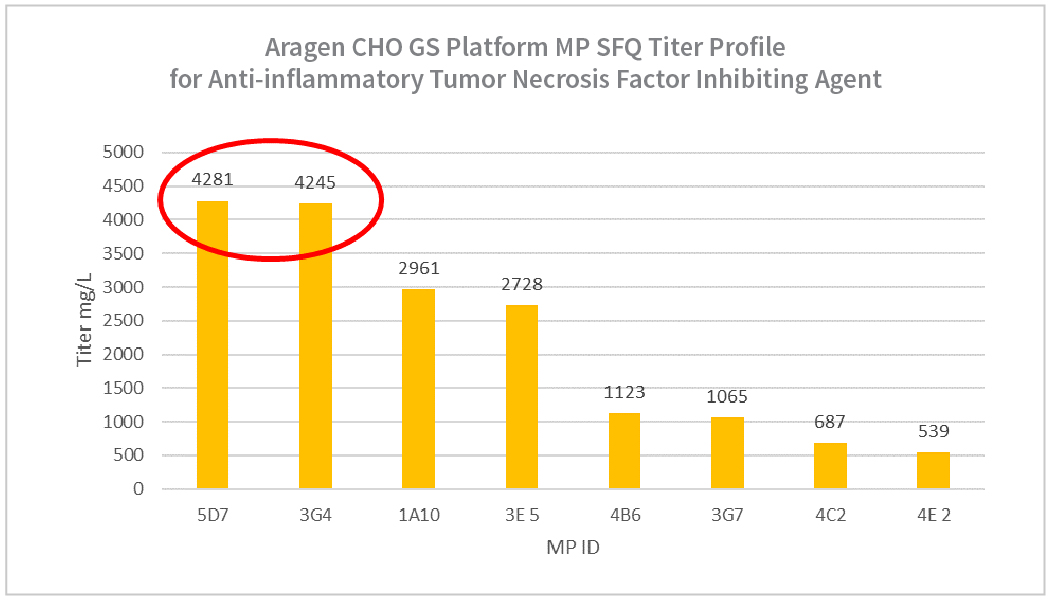
| Summary Table for Aragen Bioscience CHO GS Anti-inflammatory Tumor Necrosis Factor Inhibiting Agent MP SFQ | |||
|---|---|---|---|
| MP ID | % Viability at Harvest | PVCD E6 c/mL | Harvest Titer (mg/L) |
| 5D7 | 91 | 22 | 4281 |
| 3G4 | 88 | 16 | 4245 |
| 1A10 | 83 | 14 | 2961 |
| 3E5 | 93 | 17 | 2728 |
| 4B6 | 84 | 13 | 1123 |
| 3G7 | 90 | 12 | 1065 |
| 4C2 | 69 | 9 | 687 |
| 4E2 | 86 | 12 | 539 |
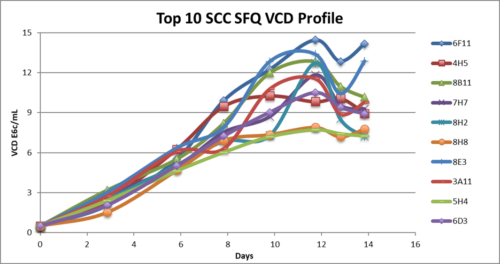
Single cell clone generation and evaluation
- The Top two MPs were used to generate SCC
- SCC in fed-batch shake flask evaluation reached up to 6g/L

| Summary Table for Aragen Bioscience CHO GS Anti-inflammatory Tumor Necrosis Factor Inhibiting Agent SSC SFQ | |||
|---|---|---|---|
| SCC ID | % Viability at Harvest | PVCD E6 c/mL | Harvest Titer (mg/L) |
| 3G4-4F8 | 86 | 18 | 5964 |
| 3G4-2C3 | 79 | 14 | 5719 |
| 5D7-3C4 | 86 | 22 | 5607 |
| 3G4-10D11 | 76 | 15 | 4977 |
| 5D7-6E10 | 95 | 28 | 4829 |
| 5D7-10A3 | 91 | 27 | 4771 |
| 3G4-9H6 | 77 | 14 | 4744 |
| 5D7-4D8 | 80 | 17 | 4721 |
| 5D7-3F11 | 89 | 25 | 4611 |
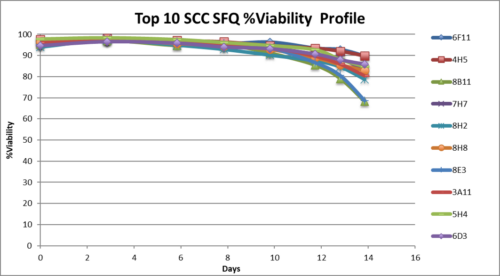
Stability study of Single cell clones
- Completed stability study on top 3 SCC form BP.
- Completed 60 days stability both with and without selection.
- High viability, good growth rate, shorter dT.
- All three SCC showed stable specific productivity up to 60 days.